Individuals with schizophrenia have selectively impaired verbal episodic memory
(1) against a background of more generalized cognitive dysfunction (see reference
2 for exception). Memory impairments appear to be due more to encoding and retrieval deficits than to long-term storage problems
(3–
5). Less consistent impairments on recognition than on free recall have led some researchers to stress the importance of encoding over retrieval
(6,
7), although a recent meta-analysis
(8) found moderate effects on recognition and large effects on free recall, suggesting that both stages of memory processing are disrupted. It is important to determine the stage of memory impairment in schizophrenia, as this information can help target pharmacological and behavioral interventions. An inherent limitation of neuropsychological testing is that encoding cannot be independently assessed because retrieval is required to obtain performance indices.
This limitation can be overcome by neuroimaging, which can be used to assess brain function during both stages of task performance. In previous positron emission tomography
(9) and functional magnetic resonance imaging studies
(10), we found that right anterior prefrontal activation during recognition was equal in patients and comparison subjects, suggesting that associated episodic retrieval mechanisms were relatively intact. In contrast, there was prominent left prefrontal underactivation and bilateral temporal-limbic overactivation during encoding and retrieval. Patients’ self-reports suggested that these frontotemporal abnormalities might have been due to differences during encoding, with patients relying on rote rehearsal rather than on more effective semantic organizational strategies
(10). To explore the role of semantic processing, encoding strategies were directly manipulated in a recent behavioral study
(11), in which we found that patients showed the same benefit as healthy comparison subjects from semantic versus perceptual encoding (i.e., levels-of-processing effect) on recognition speed and accuracy. This study demonstrated that providing a semantic strategy improves patients’ performance. The purpose of the current study was to determine if providing this strategy also normalizes brain function during both stages of memory processing, or if there are residual abnormalities that cannot be explained by encoding strategies.
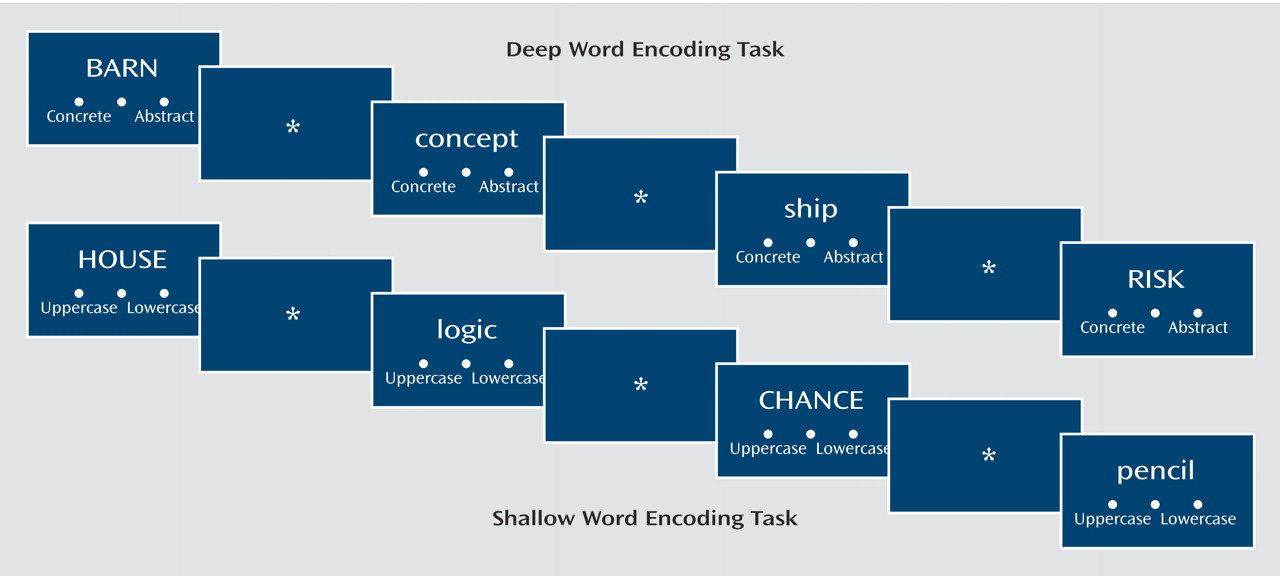
In the current study, we again employed a levels-of-processing technique. In this paradigm, Craik and Lockhart
(12) found that as information processing moves from shallow perceptual processing to more elaborative semantic-associative encoding, the strength of the memory trace increases. This increase results in a levels-of-processing effect in which retrieval is better when it follows deep processing. Because this paradigm provides participants with explicit encoding instructions, the need to generate organizational strategies is reduced. Although the paradigm can be used with verbal and nonverbal stimuli, the current study used words, and participants were required to determine if the words were presented in uppercase or lowercase letters (shallow) or if words were concrete or abstract (deep). Functional neuroimaging in healthy volunteers has demonstrated that deep versus shallow word processing is accompanied by increased prefrontal and hippocampal activation, with left ventrolateral prefrontal effects during encoding and right anterior prefrontal activation during retrieval
(13–
15).
Imaging of episodic memory in schizophrenia has revealed abnormalities in many of these same prefrontal and hippocampal regions
(16). Levels-of-processing paradigms are well suited to test the hypothesis that frontotemporal abnormalities during memory performance may be due to patients’ failure to adopt a semantic encoding strategy. However, relatively few experiments have been performed. Heckers and colleagues
(17,
18) asked patients to study word lists under shallow and deep conditions and measured blood flow during cued-recall word-stem completion. Although recall was lower in patients, both groups benefited similarly from deep encoding. Between-group comparisons revealed greater right hippocampal activation in the comparison subjects and greater prefrontal activation in the patients. Hippocampal response was blunted because of patient overactivation during baseline and shallow retrieval. A subsequent functional magnetic resonance imaging (fMRI) study examined participants during encoding and tested recognition outside the magnet
(19). Although both groups in this study benefited equally from deep processing, the patients showed decreased left prefrontal and increased left superior temporal activation for deep versus shallow words. Thus, there is evidence of both under- and overactivation of temporal-limbic and prefrontal cortex regions depending on stage of processing, and no study has examined encoding and retrieval in the same group of subjects.
The subjects in the current study underwent fMRI during word encoding and recognition in a levels-of-processing paradigm. As noted previously, when we examined this paradigm behaviorally
(11), we found unimpaired levels-of-processing effects on recognition accuracy, suggesting that semantic processing was sufficiently intact for patients to benefit from organizational cues. This background provided support for our first hypothesis that left ventrolateral prefrontal activation to deep versus shallow words is unimpaired in patients during word encoding. Based on previous findings
(9–
11), we also hypothesized that patients do not show impairments in the right anterior prefrontal cortex during retrieval of words that had undergone deep versus shallow processing. If these hypotheses were supported and residual differences in temporal-limbic function during encoding or recognition were found, these results would suggest the presence of other information processing abnormalities that are not due to top-down frontally mediated organizational processes.
Discussion
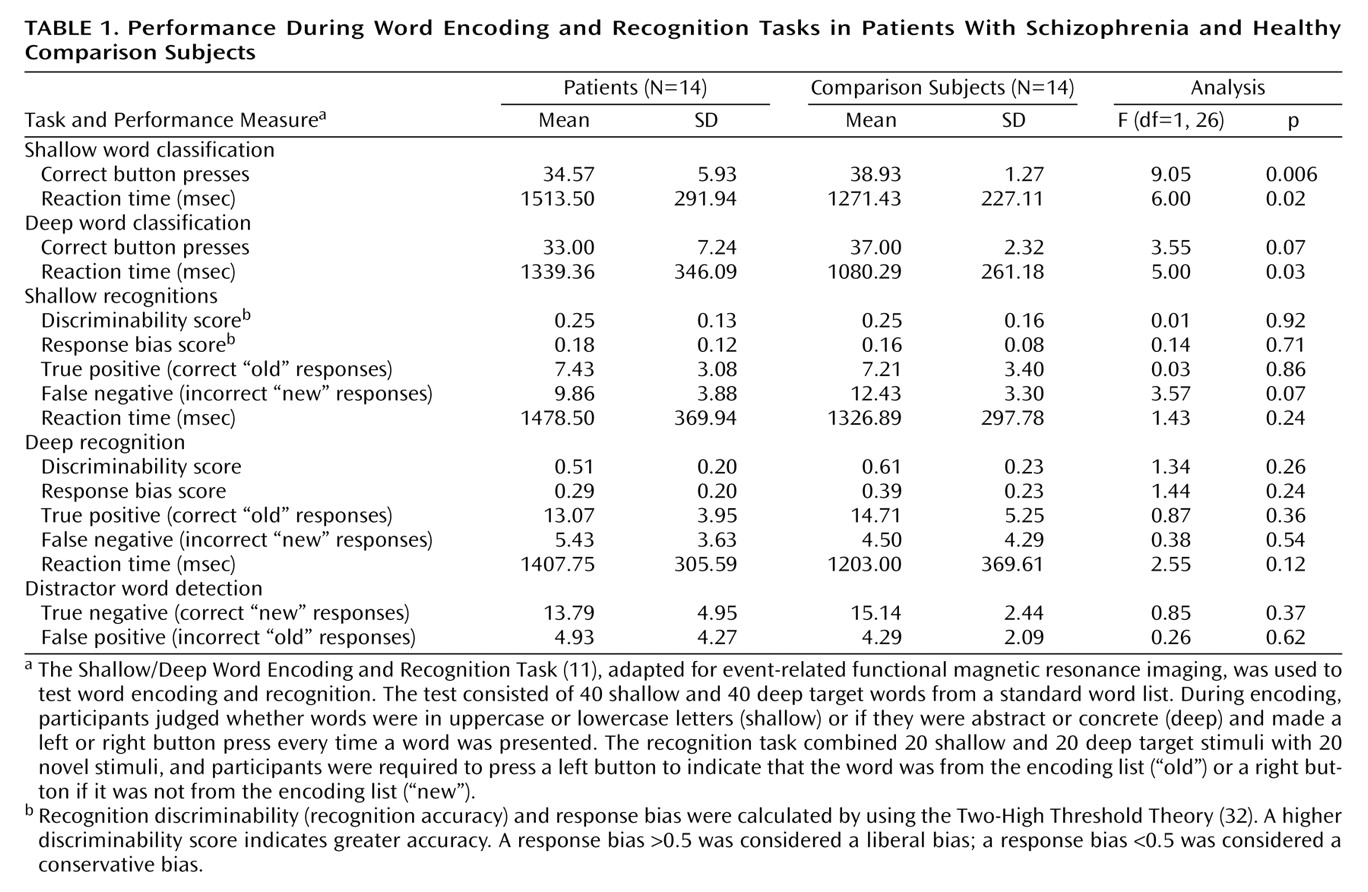
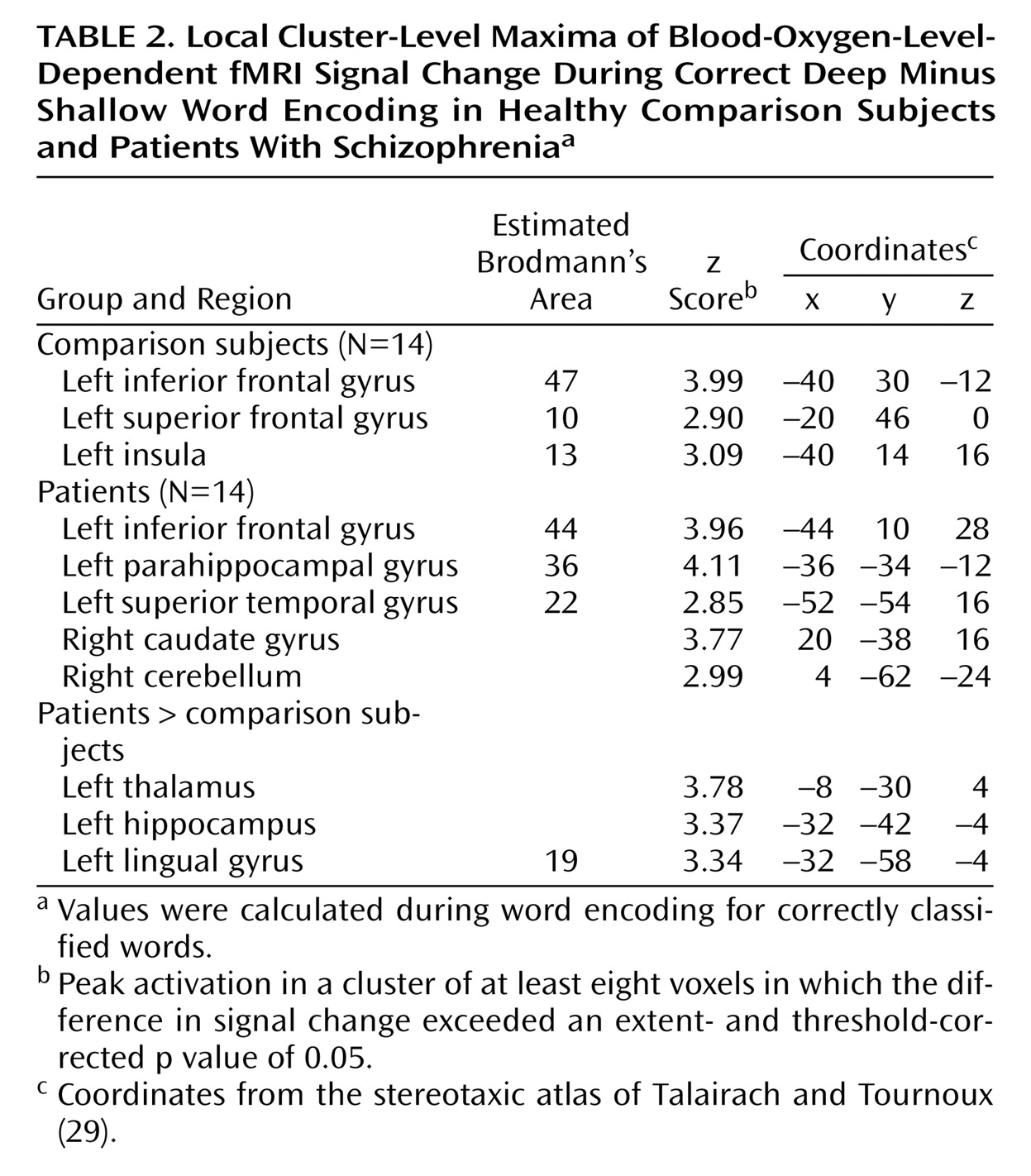
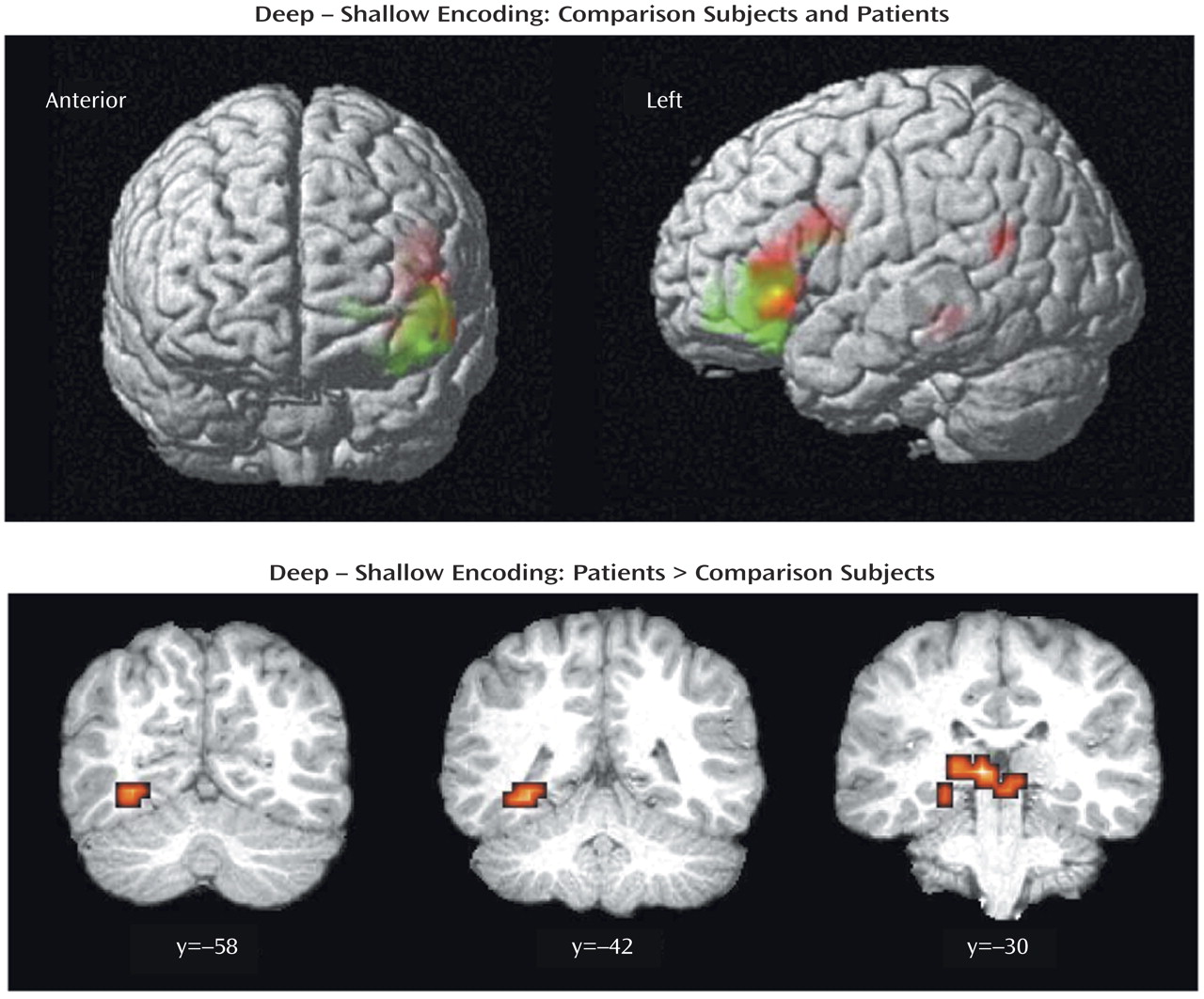
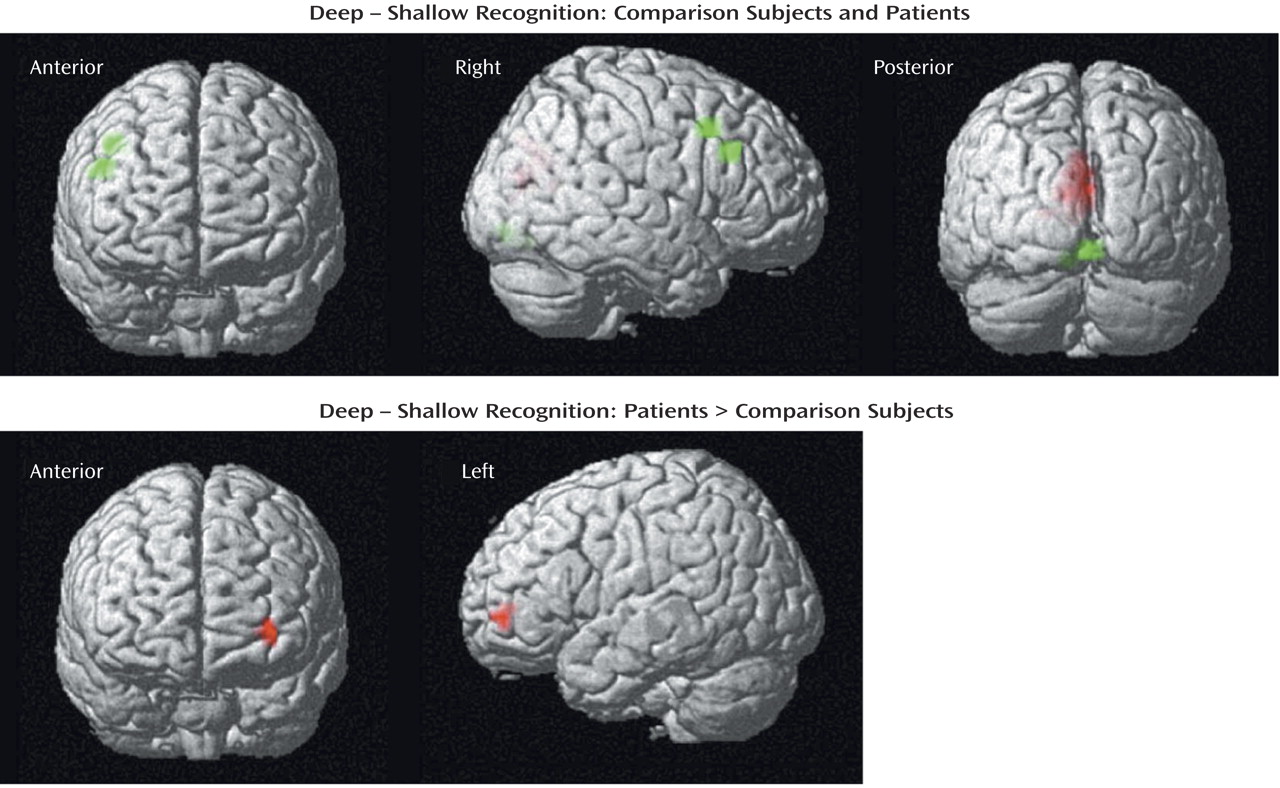
The current results supported our first hypothesis and showed that when organizational demands are reduced, patients can engage the left ventrolateral prefrontal cortex (Brodmann’s area 47) during semantic versus perceptual encoding and produce a normal levels-of-processing effect on word recognition. As reported previously
(11), words were recognized more quickly and accurately after deep processing in both groups. Although no differences were found between groups in left ventrolateral prefrontal cortex function, the activation patterns were not identical, and our second hypothesis was not fully supported. During encoding, the patients were slower and less accurate in classifying words, and they showed left hemispheric overactivation in the thalamus, hippocampus, and lingual gyrus. During recognition, activity in the right prefrontal cortex was above threshold only for the comparison subjects, although no significant difference between groups was found when the results for the two groups were directly contrasted. The patients also showed overactivation of the left frontal pole during retrieval. These results indicate that patients can engage the left prefrontal cortex during semantic processing and argue against a static lesion model of hypofrontality
(38). However, in the patients, residual patterns of temporal-limbic overactivation during encoding and less robust right anterior prefrontal activation during retrieval suggest the presence of other information processing difficulties during both stages of memory processing that are independent of top-down frontally mediated semantic organizational control.
The left ventrolateral prefrontal cortex was activated during encoding in both groups. This region is consistently associated with depth of processing
(39). As noted by Kubicki and colleagues
(19), primate studies show that the ventrolateral prefrontal cortex receives its largest input from posterior and temporal lobe regions
(40), supporting its role in maintenance and activation of episodic information in working memory. However, the notion that the region’s role is limited to semantic working memory was recently challenged by Otten and colleagues
(15), who found the expected left ventrolateral prefrontal cortex activation for deep versus shallow encoding. However, they also found that ventrolateral prefrontal cortex activation predicted successful word recognition regardless of encoding condition. These findings suggest a more general role for the ventrolateral prefrontal cortex. In the current study we did not investigate retrieval success because too few events would have resulted if we followed recommendations
(15) to limit analysis to words that were recognized with a high level of confidence. However, in the context of the analyses that were performed, the results seem to support the conclusion that the left ventrolateral prefrontal cortex facilitates depth-of-processing in both groups by activating and maintaining semantic representations in working memory.
Left hemisphere overactivation during encoding suggests a residual disturbance in distributed mnemonic or semantic processing in schizophrenia. Group comparisons revealed overactivation in the patients in the left hemisphere in the thalamus, hippocampus, and lingual gyrus during encoding and in the frontal pole during retrieval. Left temporal hyperactivity has been documented in previous studies of schizophrenia
(41), and evidence of more diffuse patient activation is consistent with previous findings
(10). In an earlier study, we speculated that parahippocampal overactivation could be explained by a model of disrupted or reversed functional connectivity of prefrontal and temporal-limbic structures
(42). It is notable that prefrontal activation was reduced, consistent with the reversal hypothesis. Similar reversals were seen in levels-of-processing studies, with lowered prefrontal and increased superior temporal activation during encoding
(19) and increased prefrontal and reduced hippocampal activity during cued recall
(17,
18).
Unlike previous studies, the current study found evidence of hippocampal overactivation during encoding despite unimpaired left prefrontal response. This dissociation of prefrontal and temporal-limbic function does not fit the classic reversal model and suggests that hippocampal hyperactivity may be independent of top-down prefrontal control. It is possible that overactivation reflects reduced inhibition of lexical networks in schizophrenia. Inclusion of left-handers does not appear to be responsible, as overactivation in the patients was also found when data for the two left-handed patients were removed. A number of studies have demonstrated increased semantic associative priming in schizophrenia, especially when symptoms of formal thought disorder are present
(43). During semantic processing, patients may have experienced increased spread of activation
(44), resulting in functional overactivation. In an exploratory analysis we directly tested the role of thought disorder by using a two-sample t test in SPM2 to contrast patients with (N=6) and without thought disorder (N=8) as measured by the SAPS (analysis available on request). We did not find any subgroup differences in the BOLD response to deep correct encoding minus shallow correct encoding in the hippocampus or other areas of patient overactivation even at a liberal significance threshold of p<0.01 (uncorrected). However, the range of thought disorder was restricted in the current group of subjects, and a more robust test of the role of thought disorder in hippocampal overactivation during semantic processing will require testing of patients with a wider range of thought disorder.
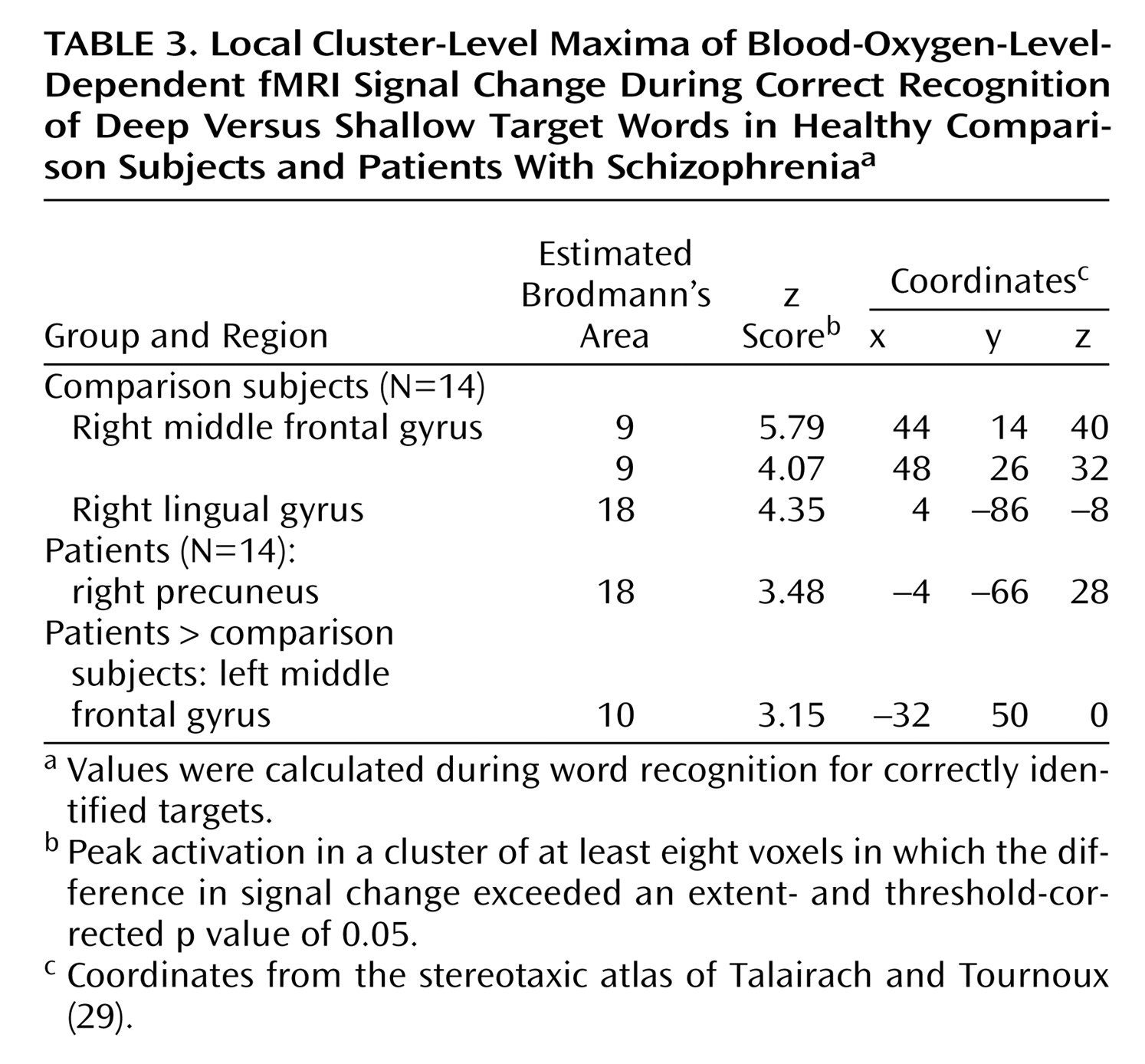
During recognition testing, semantic processing appeared sufficient to support robust levels-of-processing effects on performance. However, activity in the right prefrontal cortex was not as robust in the patients, suggesting possible differences in retrieval strategies. Although both groups activated the right visual association areas, only the comparison subjects produced above-threshold effects in a right prefrontal region traditionally associated with episodic retrieval
(45). This group difference appeared to be a matter of degree, as right prefrontal activity was seen in the patients at a lower threshold and no difference between groups was found in a direct contrast. Nevertheless, the less robust right prefrontal activation in the patients may be due to the patients’ relying more on a sense of familiarity than on episodic knowledge during retrieval
(46). The increased spread of activation in the patients during semantic encoding might result in a less secure memory trace, leading to greater reliance on familiarity effects.
Previously we found a more conservative response bias in patients after deep encoding and concluded that this response may reflect problems in source monitoring
(11). Although we did not replicate this finding in the current study, the patients’ response bias for deep words was in a more conservative direction. Lack of group differences may have been due to reduced power because of the smaller number of subjects. Analysis of fMRI data acquired with source memory probes will permit direct examination of memory trace and retrieval strategies.
Several concerns raised by the current study should be considered. Unlike previous studies
(17,
18), we did not find evidence of hippocampal activation in the comparison subjects. Our ability to detect hippocampal overactivation in the patients argues against a problem in fMRI sensitivity. A likely explanation is that the recognition task did not require subjects to make a conscious recollection of the memory event, which likely reduced hippocampal demands
(47). A future study employing recognition and recall probes may help resolve this issue. Our results were also inconsistent with those of a study that found attenuated left ventrolateral prefrontal cortex response in patients with schizophrenia
(19). It is unclear whether this discrepancy is due to the patients’ heterogeneity or to differences in experimental design and analysis procedures. One design difference is that the previous study required more frequent alternation between encoding conditions. Greater response alternation demands may have increased frontal lobe activation in the patients during shallow encoding and accounted for the lack of differences between conditions.
We studied patients who were taking medication, and the patients were more closely matched to comparison subjects than in many of our previous studies. These factors limit the generalizability of the results to more acutely ill and thought-disordered patients. However, the approach also helped control potential confounding variables and ensured that the patients were able to complete the study. It is unlikely that medication explains the current fMRI results, as previous studies have not found medication effects on prefrontal or mesial temporal function
(10,
17,
18) (see reference
48 for exception). However, in an exploratory analysis, a subgroup of patients receiving typical neuroleptics (N=3) did appear to have more severe negative symptoms and worse performance during semantic encoding and recognition, compared to patients receiving atypical neuroleptics (N=11). This finding is consistent with the notion that atypical neuroleptics may have beneficial cognitive effects
(37) and argues for larger-scale studies that can test the effect of typical versus atypical medications on fMRI activation. Finally, the finding that recognition memory and left prefrontal function can be restored in clinically stable patients holds promise for remediation efforts. A worthy goal is to develop interventions to facilitate patients’ use of organizational strategies during initial encoding and to test if these interventions improve memory and functional outcome.