Cognitive impairment is now seen as an inherent feature of schizophrenia
(1) and has been documented in many different domains, including attention
(2), executive functioning
(3), episodic memory
(4), verbal skills
(5), and processing speed
(6). Cognitive deficits may represent a core pathophysiological feature of the illness because at the time of the first psychotic episode these impairments are very similar in profile and severity to those seen in patients with more chronic illness
(7). These deficits are apparently present even before the onset of the first psychotic features of the illness
(8) and appear to worsen slightly as illness onset approaches
(9).
A cross-sectional association between cognitive deficits and poor social and occupational outcomes has been demonstrated
(10). In patients with an established illness, the correlation between cognitive and functional impairments is consistent across wide variations in the severity of lifetime functional impairment
(11). It has been argued recently
(12) that treatment of cognitive deficits, through a variety of means, has the potential to change the functional outcome of the illness, although this remains to be demonstrated directly. This possibility is important because conventional antipsychotic treatments appear not to have a particularly beneficial impact on aspects of functional outcome such as independent living
(13).
Early treatment of cognitive impairments may have important implications because disability may develop early in the course of illness, often within 6 months of initial diagnosis
(14). Treatment with novel antipsychotic medications has been suggested to be superior to first-generation treatments for enhancement of cognition in a variety of populations
(15,
16), such as first-episode patients
(17), patients with chronic illness
(18,
19), treatment-refractory patients
(20), and elderly patients
(21). These data suggest that risperidone, olanzapine, quetiapine, ziprasidone, and clozapine are associated with improvements in cognitive performance.
Several questions have been raised about some of these findings, however. Many studies used research methods that are susceptible to biases, such as open-label designs. Other studies have used comparison doses of conventional medications that were high relative to the doses of the novel antipsychotics
(16). Green et al.
(22) found that there was little difference in cognitive change between lower doses of haloperidol and relatively high doses of risperidone in chronically ill patients. Finally, some of the studies did not consider the previous medications patients had been taking before they were randomly assigned to study medications. In many studies, patients who were poor responders to one medication and selected for the trial could be assigned to receive the same medications, which may be a source of bias.
This article presents the results of a study that is not subject to most of those concerns. Patients who were in the early stages of psychosis, who met criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder, and who had received less than 12 weeks of lifetime treatment with antipsychotic medications were included. These patients were randomly assigned to receive treatment with low doses of risperidone or haloperidol. The patients were enrolled in a long-term treatment protocol and were examined with a battery of cognitive assessments previously shown to be relevant to functional outcome. Thus, participants did not have extensive previous treatment, they were treated with low doses of medication in a randomized double-blind protocol, and the data were examined with intent-to-treat analyses. The study continued for up to 5 years (until the last enrolled participant completed 2 years of treatment), but this first report presents the cognitive functioning changes from baseline to 3 months of treatment with the two target medications.
Method
Subjects
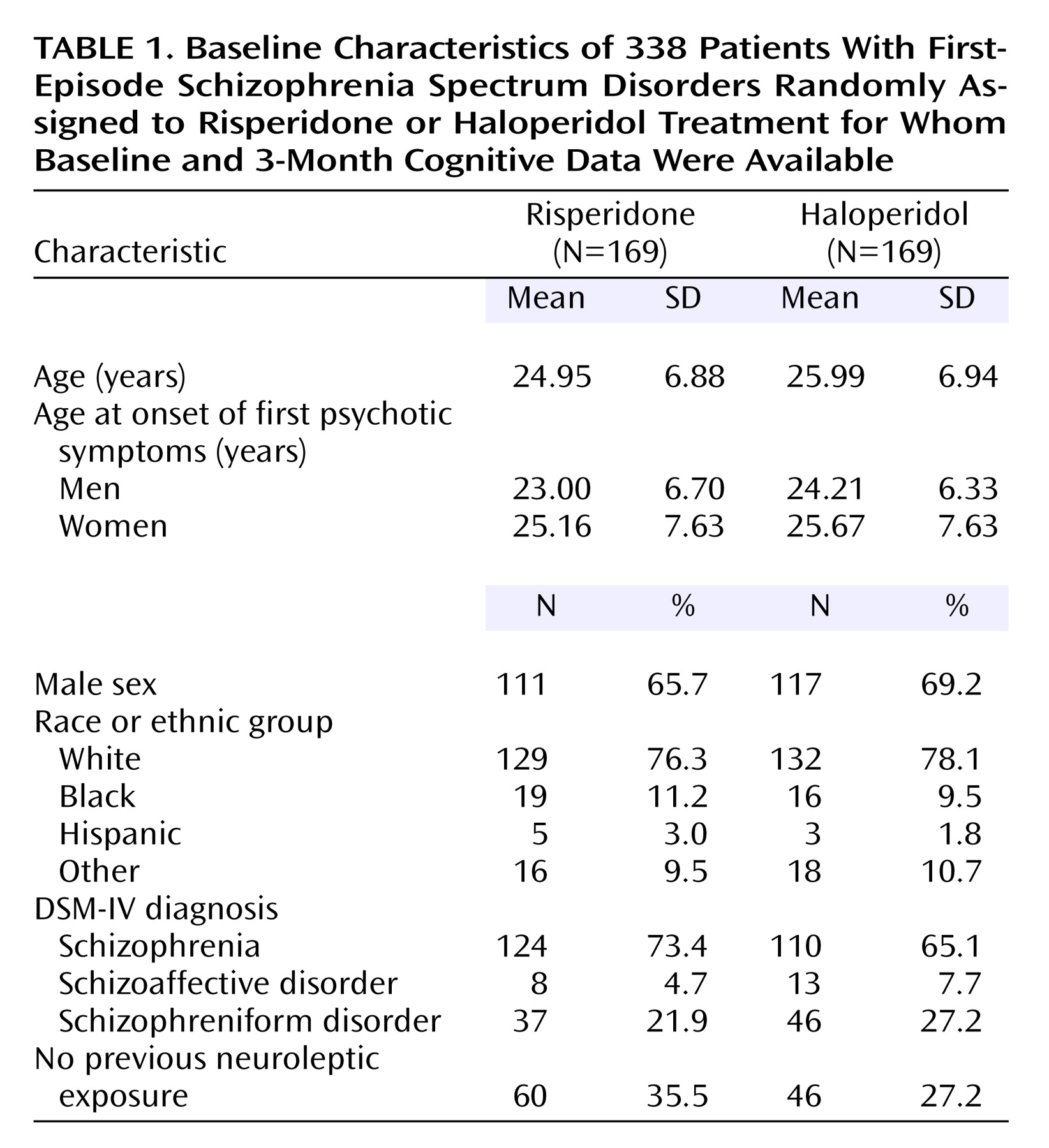
The current randomized controlled trial enrolled patients experiencing an early episode of schizophrenia in 12 countries. The characteristics of the patients randomly assigned to the two treatment groups were similar; there were no significant differences in sex, race or ethnicity, age, diagnosis, or previous neuroleptic treatment (
Table 1). Diagnostic information was collected by using the Structured Clinical Interview for DSM-IV. About half of the subjects had a diagnosis of schizophrenia, 68% were men, and their mean age was in the 20s.
Patients provided written informed consent to receive double-blind treatment with risperidone or haloperidol for a period of 2 or more years. They were informed that they could terminate their participation at any time for any reason. Patients were excluded from participation if they were unable to read their native language; had a history of head trauma, seizure disorder, or other neurological condition; had a diagnosis of mental retardation; had experienced previous psychotic episodes; or if there was any suggestion that the presence of any other diagnosis (e.g., substance abuse) would exclude a diagnosis of schizophrenia. The study was conducted in accordance with good clinical practice after it was approved by the local institutional review boards.
Subjects were recruited in the United States, Canada, United Kingdom, South Africa, Australia, New Zealand, Finland, Germany, Austria, France, the Netherlands, and Israel. To participate in the cognitive assessments, patients were required to be native speakers of the language in which they were tested, although nonnative speakers were involved in the other assessments.
Treatment
Patients were randomly assigned to receive double-blind treatment with either risperidone or haloperidol on a one-to-one randomization basis with equivalent dosing strategies. Subjects were started on 1 mg/day of the study drug and titrated up to 4 mg/day or, in exceptional cases, to a maximum of 8 mg/day. Patients were treated with trial medication for a median of 192 days (range=2 to 1,502) in the risperidone group and for a median of 218 days (range=1 to 1,514) in the haloperidol group (z=0.116, p=0.90, Mann-Whitney test). The mean modal total doses were 3.3 mg/day of risperidone and 2.9 mg/day of haloperidol.
We have reported the efficacy and safety results elsewhere
(23). Briefly, we found that scores on the Positive and Negative Syndrome Scale subscales
(24) and Clinical Global Impression (CGI) severity and change scales
(25) improved significantly over baseline and that there were no significant differences between the treatment groups. Three-quarters of the patients achieved initial clinical improvement, defined as a reduction greater than 20% in total score on the Positive and Negative Syndrome Scale. However, among those who achieved clinical improvement, Kaplan-Meier survival analysis revealed that 42% of the risperidone group relapsed, compared with 55% of the haloperidol group. The median time to relapse was 466 days for risperidone and 205 days for haloperidol (log rank=7.10, p=0.008). There were significantly more extrapyramidal side effects and use of adjunctive medications in the haloperidol group and greater prolactin elevation in the risperidone group. Relatively fewer patients in the risperidone group received concomitant medications as treatments for extrapyramidal side effects. These differences were statistically significant for β-blocking agents (p<0.02) and showed a nonsignificant difference for anticholinergics (p<0.07) and benzodiazepines (p<0.08). There was less weight gain with haloperidol initially but no significant differences between groups at endpoint.
Cognitive Battery
The cognitive battery was administered to all patients at baseline and at month 3. Research assistants trained by one of us (P.D.H.) performed all of the assessments. Training sessions occurred in small group settings at the local sites, as well as in regional meetings. Testers were required to be native speakers of the local language and fluent in English for training purposes. All were required to perform valid testing, as certified by case record form review, before any patients were examined. Case record forms were reviewed by study monitors who were trained by one of us (P.D.H.). Forms with errors were returned for correction before the blind was broken on the study.
A systematic procedure (described elsewhere
[26]) was used to ensure that the translations of all of the tests were valid. A local expert was identified in each country where English was not the language of assessment. This expert assisted in the translation of the instructions and test stimuli with these translations, which were then confirmed by back-translation. The tests are described in the order in which they were administered to subjects.
Wechsler Memory Scale—Revised Visual Reproduction Subtest (27)
This is a test of memory for nonverbal stimuli measuring visuomotor speed. For this protocol, the critical dependent variables were immediate and delayed memory raw scores.
Rey Auditory Verbal Learning Test (28)
This is a test of verbal learning and memory that is in common clinical use. The critical dependent measures for this protocol are list A total learning on trials 1–5 (reflecting cumulative learning with exposure and practice), recognition discrimination, and the long-delay recall score.
Continuous Performance Test, Identical Pairs Four-Digit Version (29)
This is a commonly used test of vigilance. The dependent variable for this test was the signal detection index, d′, which is the response sensitivity for discrimination of target and nontarget stimuli over 450 test trials.
Verbal fluency examinations (30)
In this part of the protocol, two separate versions of verbal fluency examinations were administered. In the fluency condition labeled “category,” subjects were asked to produce the names of as many different animals as possible within a 1-minute period, followed by as many fruits as possible and then as many vegetables. In the fluency condition labeled “letter,” subjects were asked to produce as many words that start with three different letters (depending on the language of assessment) for 1 minute each, excluding proper names. Critical dependent variables were total scores for letter and category verbal fluency.
WAIS-R Digit Symbol (31)
This is a test of psychomotor speed and attention. The critical dependent variable was the age-corrected scaled score.
Wisconsin Card Sorting Test (32)
This is a commonly used test of executive functioning, measuring cognitive flexibility, maintenance of a cognitive set, and working memory. In these analyses, the critical dependent variables are the number of categories completed and the total number of errors made.
Efficacy Evaluation
As described elsewhere in detail
(23), patients were assessed for treatment safety and efficacy. Of relevance to the current report, patients were assessed with the Positive and Negative Syndrome Scale
(24) by raters who were trained in small-group training sessions, with certification occurring afterwards. All raters were required to meet reliability criteria before they were allowed to perform ratings that entered the database. Patients were assessed on the Positive and Negative Syndrome Scale weekly during the first 4 weeks of the trial and then every 4 weeks for the next 5 months. Efficacy and cognitive evaluations presented in this report were performed on the same day.
Statistical Analysis
An intent-to-treat analysis was done on the 533 patients who were randomly assigned to either haloperidol or risperidone, with a focus on the 3-month reassessment of the patients. Data on 24 patients were excluded from the intent-to-treat analysis: three patients assigned to risperidone and one patient assigned to haloperidol were excluded because they did not receive study medication, and 11 patients assigned to risperidone and 10 assigned to haloperidol, all from the same site, were excluded before the blind was broken because of violations of good clinical practice.
Before analysis, composite cognitive scores were created in two ways. First, performance on all variables was standardized in the entire baseline study group, and z scores were created for each critical variable (excluding Wisconsin Card Sorting Test total number of errors). These standard scores were then averaged across all of the dependent variables to create a composite score. Second, to develop an empirically derived overall score and to avoid weighting cognitive scores that were not correlated with the other scores, a principal components analysis was also computed (see Keefe et al.
[17] for a recent example of this data analysis strategy).
Analysis of covariance (ANCOVA) controlling for age and education with treatment group as a factor was used to compare differences in test scores between the treatment groups at baseline. Change from baseline to month 3 was examined separately for each treatment group by using one-sample t tests. Differences between the treatment groups in change from baseline on each individual measure and the two summary measures (i.e., averaged z scores and first principal component) were tested by using ANCOVA models controlling for baseline values, age, and education.
Results
Data Available for Analysis
Among the patients who were eligible for reassessment, data were available for one or more measures at the 3-month assessment, or at earlier termination, for 338 patients (exactly 169 patients in each treatment group). There were no significant differences in reasons for discontinuation across the medications
(23). No differences in the rate of discontinuation across all reasons were statistically significant. Patients for whom baseline and follow-up data were available were compared with those for whom only baseline data were available on all of the demographic, clinical, and cognitive variables. The only significant demographic difference was that the percentage of male patients in the group with baseline data only (77%) was slightly higher than in the group with baseline and follow-up data (68%) (χ
2=4.70, df=1, p=0.03). The only statistically significant difference in the clinical and cognitive variables was that the patients with only baseline data produced significantly more total words on category and phonological fluency combined (t=2.18, df=503, p=0.03). Patients who were not tested after the baseline assessment had higher baseline scores on the Positive and Negative Symptom Scale (mean=69.27, SD=21.31) than those who had 3-month testing scores (mean=65.06, SD=20.35).
There were no significant differences on the composite cognitive measure at baseline between patients who had (N=232) and did not have (N=106) previous antipsychotic treatment (F=0.61, df=1, 336, p=0.43) and no significant difference on change from baseline to month 3 for this variable in an ANCOVA controlling for baseline value between those with and without previous neuroleptic treatment (F=0.77, df=1, 335, p=0.38). There was also no significant interaction between treatment group and previous neuroleptic exposure (F=0.70, df=1, 333, p=0.41).
Change From Baseline Within Treatment Groups
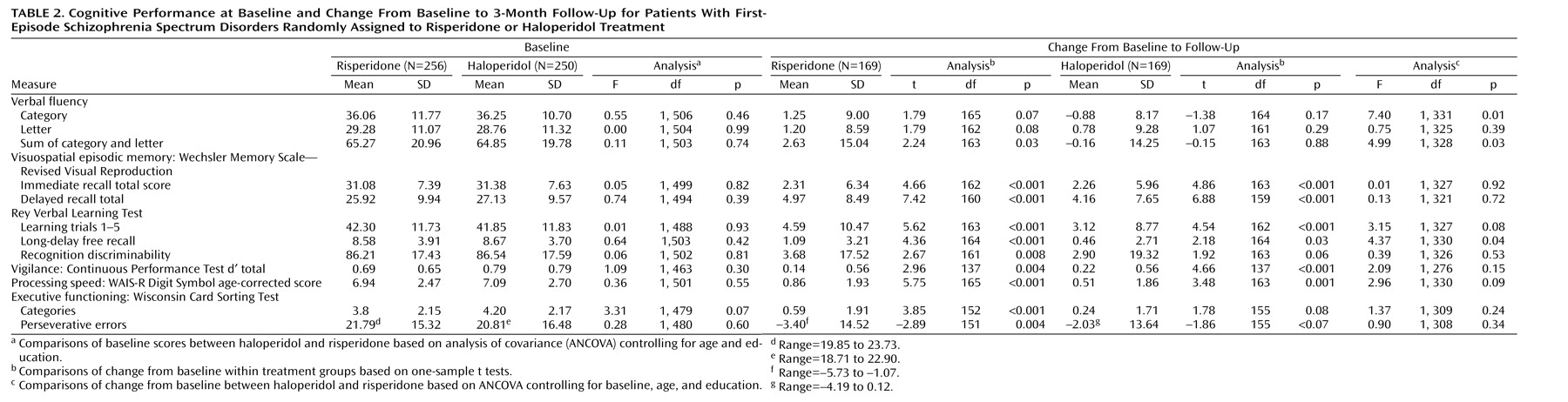
Table 2 presents baseline scores and change from baseline to the endpoint for each treatment group and one-sample t tests testing whether change in each group was significantly greater than zero. There were no statistically significant differences in any of the scores at baseline between the groups, based on post hoc tests corrected with ANCOVA for age and education (all p>0.30).
At month 3 endpoint, there was significant (p<0.05) improvement in the risperidone group for all measures except for category verbal fluency and letter verbal fluency. For haloperidol-treated patients, statistically significant improvements from baseline were noted on all measures with the exception of category verbal fluency, letter verbal fluency, Rey Auditory Verbal Learning Test recognition discriminability, and Wisconsin Card Sorting Test categories and perseverative errors.
Comparison of Treatment Groups
Comparisons between groups based on ANCOVA models showed significantly more improvement in the risperidone group than the haloperidol group on the summary cognitive score (F=4.25, df=1, 338, p=0.04, effect size=0.2). The same results were found when the first principal component was used as the outcome measure: a significant difference between the treatment groups favoring more change in the risperidone group (F=4.60, df=1, 331, p=0.03, effect size=0.2). On the specific tests there was also significantly more improvement with risperidone treatment on category verbal fluency and long-delay free recall.
Correlations With Other Change in Symptoms
To examine correlations between treatment-related changes in the cognitive variables and changes in symptoms, simultaneous-entry regression analyses regressed all of the cognitive change scores on the Positive and Negative Syndrome Scale positive, negative, and total change scores, which were calculated separately in the two treatment groups. The overall regression analysis was significant only for Positive and Negative Syndrome Scale total change scores and only in the haloperidol group (R2=0.18, N=338, p<0.05).
Discussion
We found that cognitive functioning improves in patients with early psychotic illness treated with haloperidol and risperidone. The number of patients included in this study was substantial, even with dropouts, and the history of previous treatment with antipsychotic medication was minimal and did not affect baseline or change scores on the cognitive measures. Thus, many of the methodological limitations that plague research in this area are absent from this study. Further, results of the treatment were consistent with those of previous research using similarly high methodological standards and meta-analyses of more methodologically limited studies. Risperidone improved more aspects of cognitive functioning than did haloperidol, and global cognitive functioning, calculated in two different ways, also demonstrated relative superiority for risperidone treatment. This level of comparative benefit was relatively small, and the clinical significance of a change of this magnitude is yet to be determined.
The aspects of cognitive functioning that were improved by the two medications are among those suggested by previous research to be related cross-sectionally to functional outcomes. The current study did not directly examine psychosocial outcomes, and the treatment-related improvements in this study are not demonstrated to have any immediate functional benefit. Low doses of both haloperidol and risperidone were used in this study, addressing the concern that major differences between conventional and atypical medications might be related to differential dosing of the conventional antipsychotic. That the dropout rates were essentially identical across the two medications during the first 3 months of the study might be attributable to this low-dose strategy.
Consistent with earlier reports of first-episode subjects treated with low doses of conventional medications, there was improvement in some domains of cognitive functioning with haloperidol treatment
(17). The current results are different from those of Green et al.
(22), who found no relative benefit for risperidone compared with haloperidol over a 2-year follow-up. That study had considerably fewer subjects (N=40 at 6 months and 33 at endpoint), and there are two additional important differences between that study and the current study: 1) the subjects had extensive treatment histories and 2) the dosing of risperidone was relatively high (6.0 mg/day). Similarly, previous studies using higher doses of risperidone with fewer subjects
(33) have also failed to detect substantial cognitive benefits of this treatment.
Robinson et al.
(34) demonstrated that conventional treatment of psychotic symptoms at the time of the first episode led to excellent treatment response in terms of reduction of psychotic symptoms. However, the typical patient receiving conventional treatments in their study experienced one or more relapses and also had relatively poor functional outcome over the next 5 years
(35,
36). Although cognitive change with conventional antipsychotic treatments in the Hillside Hospital Treatment Study has not been presented yet, it is clear from several publications
(37,
38) that the patients in this study had considerable cognitive impairment at the time of their first admission to treatment. Better cognitive functioning predicted greater recovery of social functioning in the Hillside Hospital Treatment Study, which has positive implications for the results of our study, which found evidence of cognitive enhancement. Other publications from our study address clinical changes and stability of symptomatic improvement over time
(23). It is clear, however, that cognitive improvement is demonstrated at the time of the first episode with a relative benefit for atypical antipsychotic treatment.
In view of the finding of an overall correlation between cognitive changes and changes in total Positive and Negative Syndrome Scale scores with haloperidol treatment (despite relatively smaller changes overall), it is possible that these cognitive changes are related to general clinical state variables and not to specific cognitive enhancement. This interpretation would be consistent with the Hillside Hospital finding that early improvement associated with conventional antipsychotic treatments did not lead to persistent improvements in functional outcome. This finding is also consistent with the results of another recent study of atypical antipsychotic treatment of early psychosis
(17), which found that clinical and cognitive changes were more strongly related in the group receiving atypical antipsychotic treatment.
Several important issues regarding the validity of these data have been addressed by previous examinations of the baseline dataset. For instance, validity of the translations of testing and the cross-national assessment modality has already been demonstrated
(26). It was found that levels of performance were very similar across languages within multilingual countries (Canada and South Africa) and that the associations between education and cognitive performance were similar in all of the countries. The group of subjects enrolled in this clinical trial was compared with a large-scale epidemiologic sample of first-episode patients previously collected (the Suffolk County Mental Health Project)
(39). Although 33% (N=59) of the epidemiologic sample did not meet inclusion criteria for the drug trial, there were no significant differences between this sample and our patients in age at onset, age, gender, and premorbid functioning. The patients in our study had more severe clinical symptoms, slightly lower CGI scores, and less formal education than those in the epidemiologic study.
Levels of treatment-related change in cognitive functioning in our patients were lower than those seen in previous reports of the beneficial effects of risperidone (and other atypical medications) in similar double-blind clinical trials
(18–
21). At the same time, patients in this study performed somewhat better at baseline than patients in those previous clinical trials. For instance, baseline Wisconsin Card Sorting Test categories in this study were 3.8 for patients randomly assigned to risperidone, in contrast to 2.8 categories in the previous study by Harvey et al.
(18). Thus, the risperidone treatment-related improvement in Wisconsin Card Sorting Test performance led patients in the current study to average 4.4 categories completed on the Wisconsin Card Sorting Test at endpoint, in contrast to 3.2 categories in the previous study. McGurk and Meltzer
(40) reported that patients with schizophrenia who were employed full-time—an excellent and atypical employment outcome—were able to complete somewhat fewer than five categories on the Wisconsin Card Sorting Test, which is very close to the level of performance seen after 3 months of treatment in this study. Similar effects were found in this study for improvements in verbal and spatial episodic memory, where posttreatment performance was close to the 35th percentile of the normal distribution (which is not in the typical impaired range) for the average patient treated. Thus, in these first-episode patients, 3-month performance was not in the substantially impaired range, which is different from the results of other studies that demonstrated significant benefits for atypical antipsychotic treatment.
Despite the methodological strengths in this design, there are some limitations in the study that require mention. The doses of medication that were administered were quite low and may not be suitable for long-term maintenance treatment. This was not a study of exclusively drug-naive patients. However, we found no significant differences between patients who were and were not drug naive in terms of either baseline cognitive scores or changes in cognitive functioning with treatment. Since this was an analysis of 3 months of treatment, measurement of change in functional indicators would be premature, although these data will be presented later for the entire duration of the 2-year planned study.
The results of this study suggest that treatment with low doses of the atypical antipsychotic risperidone results in improvements in cognitive functioning that are somewhat greater than those seen with the conventional medication haloperidol. As noted, functional impairments and related disability are the central targets of interventions aimed at cognitive deficits in schizophrenia, and more research will be required to estimate the relative benefits, if any, associated with this intervention. Given the other findings from this study
(23) suggesting equivalence in short-term efficacy and superiority of the atypical medication risperidone compared with low-dose haloperidol in relapse prevention and in extrapyramidal side effects, these data suggest that modest benefits in cognitive functioning are also associated with treatment with risperidone.