Investigators who contribute to clinical trials often receive funding, either directly or indirectly, from sponsors with an interest in the outcome and reporting of these trials
(1,
2). Such a relationship may create conflict of interest for these authors, in which their interest in an objective description of outcomes competes with their obligation, perceived or real, to the sponsor
(3). This concern may be more than hypothetical: industry-sponsored trials may be more likely to report favorable outcomes
(4,
5), raising the possibility of influence on study design or publication bias. To address this potential bias, journals typically require disclosure of conflict of interest by authors, although journal policies on disclosure have been suggested to be inconsistent
(6) and prone to abuse
(7).
The potential consequences of financial conflict of interest in medicine as a whole have raised substantial concern in both the medical literature
(8–
10) and the lay press
(11,
12). However, apart from recent editorials
(13,
14), the prevalence and implications of conflict of interest in psychiatry have received relatively little attention. This is particularly notable given the extent of industry involvement in drug development in psychiatry, the rapid growth in pharmacotherapies in psychiatry approved by the Food and Drug Administration, and recent calls for the establishment of a clinical trial registry to ensure the fair reporting of the results of clinical trials
(12).
Therefore, we attempted to quantify the extent of industry sponsorship and financial conflict of interest in reports of clinical trials in the four general psychiatric journals with the greatest citation impact factors that commonly publish such studies. We also assessed the possible relationship between such conflict and study design and reporting.
Method
A MEDLINE search was conducted by using the term “clinical trial” and limited to articles published in the American Journal of Psychiatry, the Archives of General Psychiatry, the Journal of Clinical Psychiatry, and the Journal of Clinical Psychopharmacology between January 2001 and December 2003. These journals were selected a priori to represent the most widely cited general psychiatric journals that publish clinical trials. The MEDLINE search was supplemented by a manual review of the tables of content of these issues. The reviewers (Y.W., C.H., and M.J.) employed a standardized template to extract data on study funding sources, author affiliations, study design and outcome, and sample sizes, with coding confirmed by a fourth reviewer (R.H.P.). A subset of articles were coded by all reviewers; kappas for all coding were greater than 0.98.
The source of study funding, as well as potential author financial conflict of interest, was determined by published disclosures. We defined author financial conflict of interest as any report of consulting or speaking fees or honoraria, stock ownership, or employment by the study sponsor. Under this definition, author conflict of interest could be present even in the absence of industry sponsorship; conversely, we did not assume that industry sponsorship automatically confers conflict of interest. The only exception to this rule occurred if the author listed his or her employment with the manufacturer of a drug but the trial sponsor was not explicitly identified. In these cases, unless stated otherwise, it was determined that the study was sponsored by the author’s employer. Although data on other funding sources, including governmental or nonindustry private sources, such as foundations were examined, primary comparisons examined industry- versus non-industry-supported studies.
Study designs were classified as to whether or not they were randomized or double blind and according to the presence or absence of a placebo and/or an active comparator. Among the randomized, placebo-controlled trials, primary study outcomes were classified as interventions superior or not superior to placebo. Studies that reported secondary analyses of primary outcomes previously reported (for example, predictors of response or meta-analyses) were excluded. However, we elected to include studies in which the primary analysis addressed secondary endpoints from previously reported trials (for example, examinations of weight gain in a clinical trial) because the intent of this study was to examine clinical trial reporting.
For most univariate comparisons, Fisher’s exact test for dichotomous measures was used. Because study size demonstrated pronounced nonnormality with a right skew, the nonparametric Wilcoxon rank sum test was used for this comparison. Logistic regression was used to examine the relationship between study parameters and the likelihood of positive outcome, with adjustment for potential confounders. All analyses used Stata 8.2 (Stata Corp., College Station, Tex.).
Results
A total of 873 studies were identified from the MEDLINE search. After a manual search of tables of content and a review of abstracts, 397 were found to meet inclusion criteria. These were drawn from the American Journal of Psychiatry (N=73, 18%), the Archives of General Psychiatry (N=25, 6%), the Journal of Clinical Psychiatry (N=198, 50%), and the Journal of Clinical Psychopharmacology (N=101, 25%). Among these, 239 (60%) were randomized, 216 (55%) were double blind, and 269 (68%) included a comparator: a placebo in 173 (44%) and an active comparator in 135 (34%).
Sources of industry support, based on statements of conflict of interest and author affiliation, were also examined: 239 (60%) reported at least partial industry support, 71 (18%) reported other private support, and 101 (25%) reported public (government or equivalent) support. In addition, 77 (19%) reported no funding support. Many studies reported multiple funding sources: industry support was included in 25 of 71 (35%) privately funded studies and 47 of 101 (47%) publicly funded studies.
Of the studies assessed, 136 (34%) included a conflict-of-interest statement. In an additional 65 studies, no conflict-of-interest statement was present, but the authors’ addresses were pharmaceutical industry offices, so author conflict of interest was inferred.
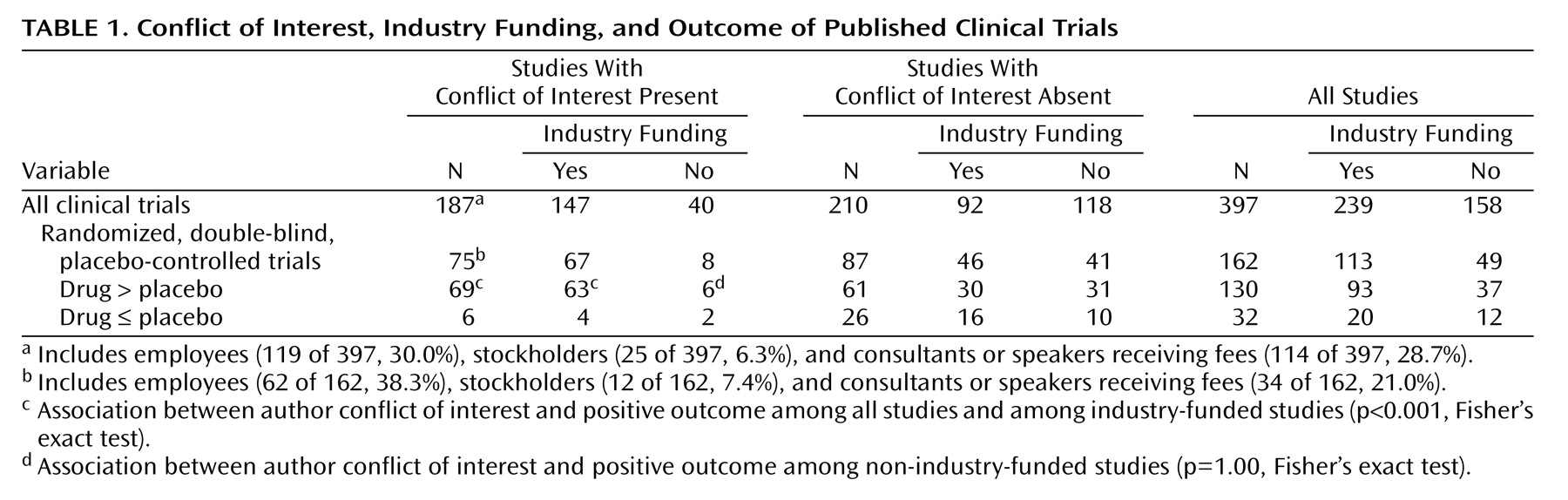
We next examined the nature of the authors’ reported conflict of interest (
Table 1). Overall, 187 studies (47%) included at least one author with potential financial conflict of interest. Studies with and without conflict of interest were generally similar in terms of design, including the use of random assignment (119 of 187, 64%, versus 120 of 210, 57%, respectively; p=0.22, Fisher’s exact test), double blinding (107 of 187, 57%, versus 109 of 210, 52%; p=0.32, Fisher’s exact test), and a placebo comparator (81 of 187, 43%, versus 92 of 210, 43%; p=1.00, Fisher’s exact test). Studies with conflict of interest were more likely to use an active comparator than studies without (75 of 187, 40%, versus 60 of 210, 29%; p<0.02, Fisher’s exact test) and included larger numbers of subjects (median sample size in study drug group: 69 [interquartile range=26–153] versus 22 [interquartile range=14–42]; z=–8.40, p<0.001).
Finally, among the 162 randomized, double-blind, placebo-controlled trials, we examined the relationship between author conflict of interest and the likelihood of a positive trial in which drug was superior to placebo (
Table 1). Author conflict of interest was significantly associated with positive trial outcomes among all studies, regardless of funding source (p<0.001, Fisher’s exact test; crude odds ratio for a positive study=4.9, 95% confidence interval [CI]=1.9–12.7), and among industry-supported studies (p<0.001, Fisher’s exact test; crude odds ratio for a positive study=8.4, 95% CI=2.6–27.3). Industry support itself was not significantly associated with a positive outcome (p=0.39, Fisher’s exact test; crude odds ratio 1.5, 95% CI=0.7–3.4). Inclusion of a term for the two higher- or lower-ranked journals, by citation impact factor, yielded similar results (adjusted odds ratio=6.1, 95% CI=2.2–17.0). Among non-industry-supported studies, no significant association was noted between author conflict of interest and study outcome (p=1.00, Fisher’s exact test).
Discussion
We found that financial conflict of interest is prevalent among clinical trials published in four widely cited general psychiatric journals. Our study identified industry funding in 60% of the trials; studies of general medical journals have revealed rates of 40% to 66%
(15–
17). The prevalence of studies with author conflict of interest in psychiatry journals (47%) was slightly higher than the rates found in general medical journals (34%–43%)
(5,
15).
The relationship between financial conflict of interest and positive outcome is consistent with prior reports in the general medical literature
(5,
17–20). To our knowledge, this is one of the first recent examinations of conflict of interest specifically in the psychiatric literature. One previous report did note differences in articles about sertraline written by medical communications companies compared to those without this affiliation
(21), providing some support for the hypothesis that industry involvement influences reporting.
We note several important limitations of this report. First, our definition of positive outcome is rather limited and concrete, relying solely on the primary outcome measure in double-blind, randomized, placebo-controlled trials. Many clinical trials provide more nuanced results or are conducted for reasons other than the establishment of efficacy or tolerability. This is particularly the case for studies that compare two pharmacotherapies directly and may be intended to establish noninferiority rather than superiority, per se, for a primary outcome. For this reason, we elected to examine outcome only in placebo-controlled trials.
We also did not explicitly measure study quality (for example, the use of quality scores of Jahad et al. in randomized, controlled trials)
(22), electing to focus instead on overall study design. Study quality is typically similar when industry funding is received
(4); in a recent examination of the dermatology literature, we found that in some cases, study quality is greater (C.S. Perlis and R.H. Perlis, unpublished manuscript).
The journals examined in this study represent the most frequently cited general psychiatric journals that commonly publish clinical trials: by 2003 Institute for Scientific Information citation impact factors, they rank first, second, sixth, and seventh
(23). Because the preponderance of trials identified were published in the
Journal of Clinical Psychiatry, it is possible that this unduly biased the results. However, excluding these trials from analysis or including a term in the regression models to account for being ranked first or second versus sixth or seventh yielded similar results.
A crucial question, and one that we are unable to address fully with the present data set, is the reason for the association between author financial conflict of interest and outcome. Conflict of interest need not imply unethical behavior or wrongdoing. For example, investigators with more experience and seniority could be expected to design better studies, which may therefore be more likely to identify positive results. These senior investigators also could be more likely to serve on advisory boards or consultancies for pharmaceutical companies. Similarly, industry sponsorship may allow larger and better-designed studies, with greater statistical power to identify significant differences if such differences exist. Indeed, the median number of subjects was larger among studies in which conflict of interest was present. Industry-funded trials would naturally examine drugs already suggested to have efficacy in earlier-stage trials. Conversely, less senior or less well funded investigators may conduct smaller pilot studies less likely to identify differences. These factors could also have contributed to our findings.
Of greater concern are other potential consequences of financial conflict of interest. It is possible that authors could be more likely to agree to study designs or reports biased toward the sponsored drug
(21,
24) or be less likely to publish negative results
(25). The latter possibility has prompted calls for the establishment of clinical trial registries to ensure that all large trials are reported fairly
(12). At a minimum, the presence of conflict of interest may necessitate greater scrutiny of results.
In sum, our results suggest that financial conflict of interest is at least as prevalent in psychiatry as in other specialties in medicine. Industry sponsorship and author conflict of interest are prevalent and do appear to affect study outcomes. Given this prevalence and the potential influence on the general psychiatric literature, it will be critical to obtain a better understanding of the ways in which industry funding or the presence of conflict of interest influences the design, conduct, and/or reporting of clinical trials. Strategies to ensure that conflict of interest is disclosed consistently and completely and registries to ensure that all clinical trials, regardless of outcome, are reported should be considered in psychiatry as in other areas of medicine.