Ketamine, a noncompetitive antagonist at the glutamatergic
N-methyl-
d-aspartate (NMDA) receptor, is currently used in human and animal medicine as an injectable anesthetic
(1,
2). Ketamine is also a controlled substance, illegally used as a recreational drug (“Special K,” “Vitamin K”). The recreational use of ketamine is prevalent at dance events
(3,
4). While no epidemiological study has specifically addressed the scope and growth rate of the nonmedical use of ketamine, the rates of emergency room visits nationwide for use of ketamine grew 20-fold between 1994 and 1999
(5).
At subanesthetic doses, ketamine induces a state of dissociation (including distortion of space, time, and body image) and feelings ranging from euphoria to detachment, an experience described by users as a mind or spiritual exploration
(6–
8). While the cognitive deficits observed during acute administration of ketamine are well documented
(7,
9,
10), very little is known of the long-term effects of repeated ketamine administration in the human brain. High incidence of psychiatric symptoms, such as recurrent hallucinations and psychotic episodes, have been described in subjects abusing phencyclidine (PCP), a more potent noncompetitive NMDA antagonist
(11). In chronic ketamine abusers, limited studies suggest the persistence of neurocognitive deficits up to 3 days after use, but these studies are compromised by the polysubstance use in these samples
(12,
13).
The animal literature suggests that repeated exposure to noncompetitive NMDA antagonists leads to sustained impairment of performance in numerous cognitive domains, such as working memory tasks (reviewed by Jentsch and Roth
[14]). These deficits induced by NMDA antagonists have been linked to reduced function of the prefrontal dopaminergic system, which plays a critical role in sustaining working memory and executive functions
(15–
19). Monkeys chronically treated with the noncompetitive antagonist MK-801 showed decreased performance on working memory tasks and decreased prefrontal dopamine levels measured with microdialysis
(20). Sustained decrease in prefrontal dopamine leads to an up-regulation of prefrontal dopamine D
1 receptors, the main dopaminergic receptor in the cortex
(21). A positron emission tomography (PET) study showed increased binding of the selective dopamine D
1 receptor radiotracer [
11C]NNC 112 in the prefrontal cortex of monkeys chronically exposed to MK-801
(20). Thus, in experimental animals, chronic exposure to NMDA antagonists has led to deficits in presynaptic dopaminergic function in the prefrontal cortex, which are associated with a compensatory up-regulation of postsynaptic dopamine D
1 receptors.
It is currently unknown if such a phenomenon (decreased prefrontal dopamine function and up-regulation of D1 receptors) is also present in humans chronically exposed to NMDA antagonists. Here, we studied the impact of repeated ketamine exposure on dorsolateral prefrontal cortex D1 receptor binding potential using PET and [11C]NNC 112 in a group of 14 recreational chronic ketamine users and 14 healthy comparison subjects matched for age, gender, race, socioeconomic status of the family of origin, and nicotine smoking.
In addition to examining potential toxic effect of repeated ketamine exposure, this study was also relevant to the pathophysiology of schizophrenia. In schizophrenia patients, we previously observed a regionally selective up-regulation of [
11C]NNC 112 binding potential in the dorsolateral prefrontal cortex
(22). A deficit in NMDA transmission has been implicated as a fundamental aspect of the pathophysiology of this illness
(23–
25). Therefore, we speculated that in schizophrenia, a chronic deficit in NMDA transmission might lead to a decrease in prefrontal dopamine function and D
1 receptor up-regulation. To document that D
1 receptor up-regulation might result from chronic NMDA antagonist exposure in humans would reinforce the biological plausibility of this model.
Results
Following a telephone screening interview, 54 potential chronic ketamine users were evaluated at the research site. Thirty-three potential participants were excluded from the study because of medical conditions (N=4), psychiatric comorbidity (N=3), abuse of other drugs (N=12), or use of ketamine below study criteria (N=14). Among the 21 subjects who met study criteria, 14 agreed to participate in the study after explanation of the nature and the risks of the study. All recruited subjects completed the study.
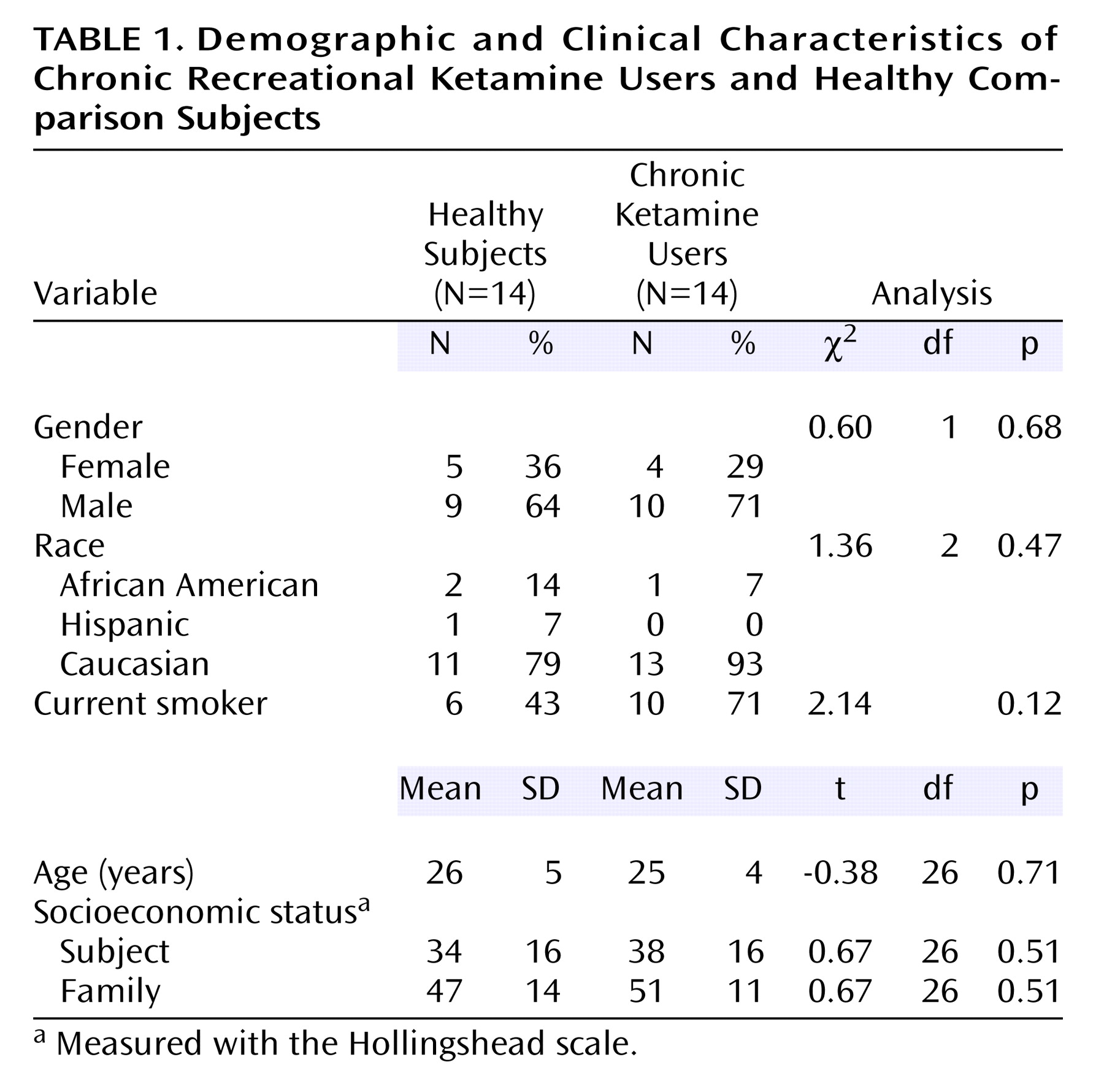
The two study groups are further described in
Table 1. The groups were predominantly composed of young Caucasian males. Chronic ketamine users had used ketamine for a mean of 4.1 previous years (SD=2.4, range=11 months to 2 years). Age did not correlate with duration of use (r
2=0.01, df=12, p=0.68). Over the preceding 3 months, the mean ketamine consumption was 2.8 vials per week (SD=1.9, range=1–7). Twelve subjects reported inhalation of ketamine as their usual mode of administration, and two subjects reported intramuscular injection. Two of the 14 chronic ketamine users also met criteria for cannabis dependence. Mean hair ketamine concentration was 32 ng/mg of hair (SD=21, range=9–70).
Groups were matched for familial socioeconomic status. No group difference was found in the socioeconomic status of the subjects. Among the chronic ketamine users, six were employed full-time, three were employed part-time, two were students, and three were unemployed. The difference between socioeconomic status of subjects and their family of origin was similar in the chronic ketamine users (mean=–10 points [SD=22] on the Hollingshead scale) and healthy subjects (mean=–13 points [SD=19]). Hence, the frequent use of ketamine did not result in a significantly lower socioeconomic status in chronic ketamine users than in healthy subjects from similar socioeconomic backgrounds.
Scan Parameters
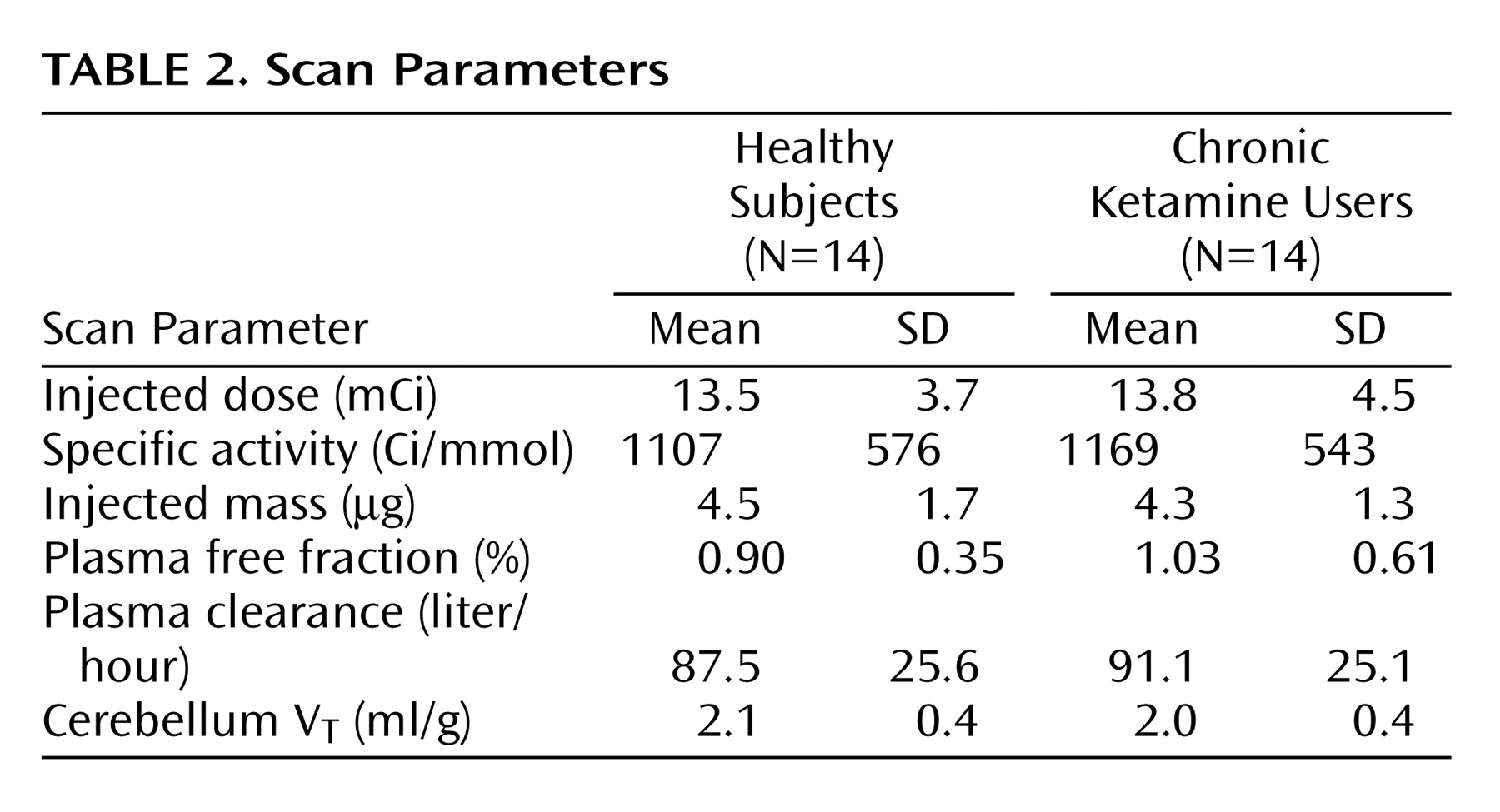
Critical PET scan parameters are listed in
Table 2. [
11C]NNC 112 injected dose, specific activity at time of injection, and injected mass did not differ between the groups. No significant between-group differences were observed in the clearance rate of [
11C]NNC 112 from the plasma compartment, in [
11C]NNC 112 plasma free fraction, or in [
11C]NNC 112 cerebellum distribution volume.
Regional Volumes
No significant between-group differences were found in dorsolateral prefrontal cortex volumes (healthy subjects: mean=49.9 cm3 [SD=16.1]; chronic ketamine users: mean=47.9 cm3 [SD=10.0]) nor in volumes of the other regions. These data indicate that the use of ketamine in the subjects included in the study did not result in detectable changes in regional brain volumes.
D1 Receptor Measurement
Region of interest analysis
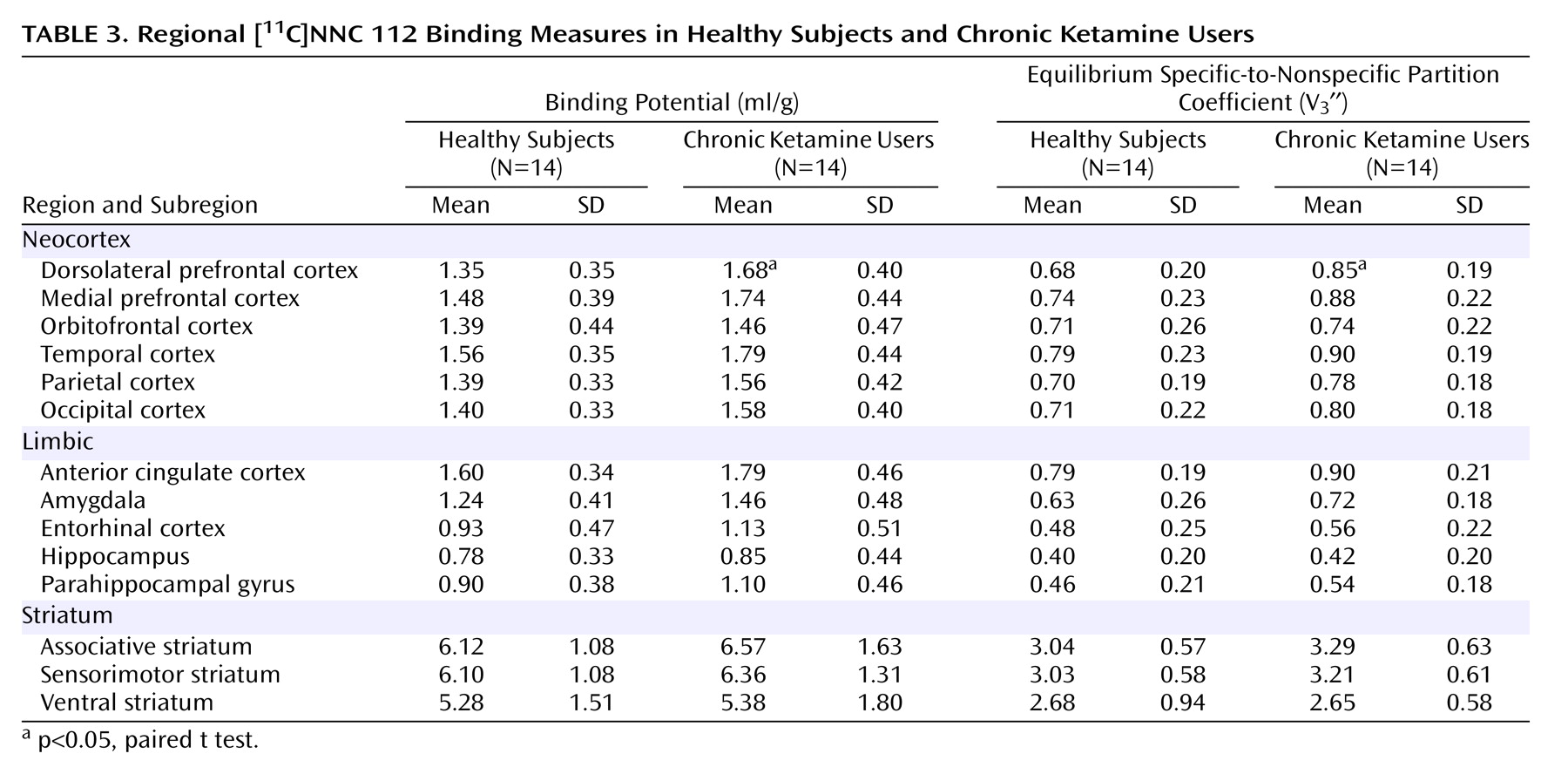
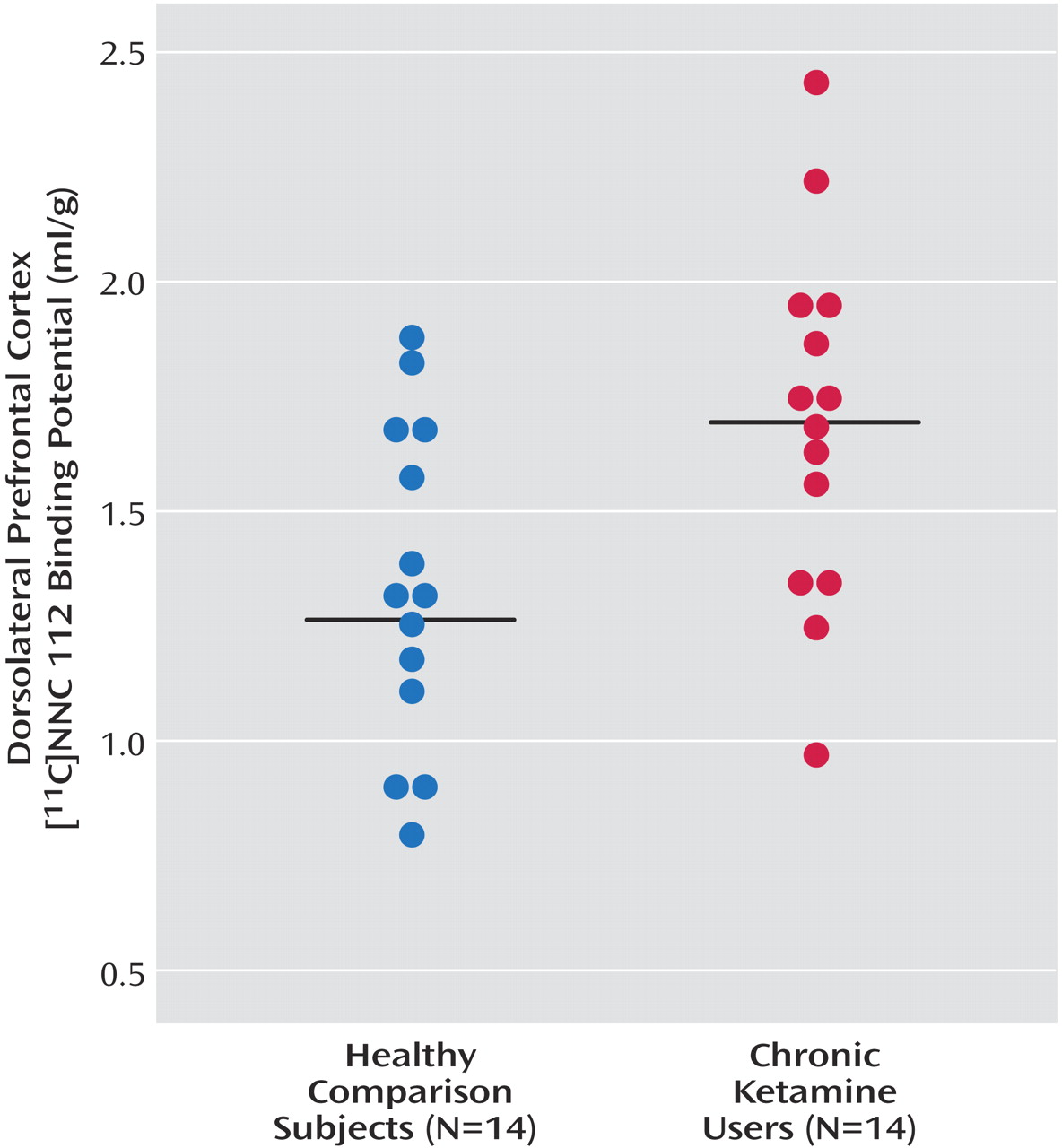
As seen in
Figure 1, dorsolateral prefrontal cortex [
11C]NNC 112 binding potential was significantly higher in chronic ketamine users compared with the healthy subjects. Similar results were obtained when V
3′′ was used as the outcome measure (healthy subjects: mean=0.68 ml/g, SD=0.20; chronic ketamine users: mean=0.85 ml/g, SD=0.22) (t=2.37,df=26, p<0.03). The two chronic ketamine users with highest dorsolateral prefrontal cortex [
11C]NNC 112 values were the subjects who reported predominant intramuscular administration. The group difference in dorsolateral prefrontal cortex [
11C]NNC 112 binding potential remained significant after exclusion of the two subjects with concomitant cannabis dependence (p=0.048). [
11C]NNC 112 binding potential and V
3′′ values in other regions are listed in
Table 3. No significant between-group differences were observed in [
11C]NNC 112 binding potential and V
3′′ in other regions examined. In the chronic ketamine users, dorsolateral prefrontal cortex [
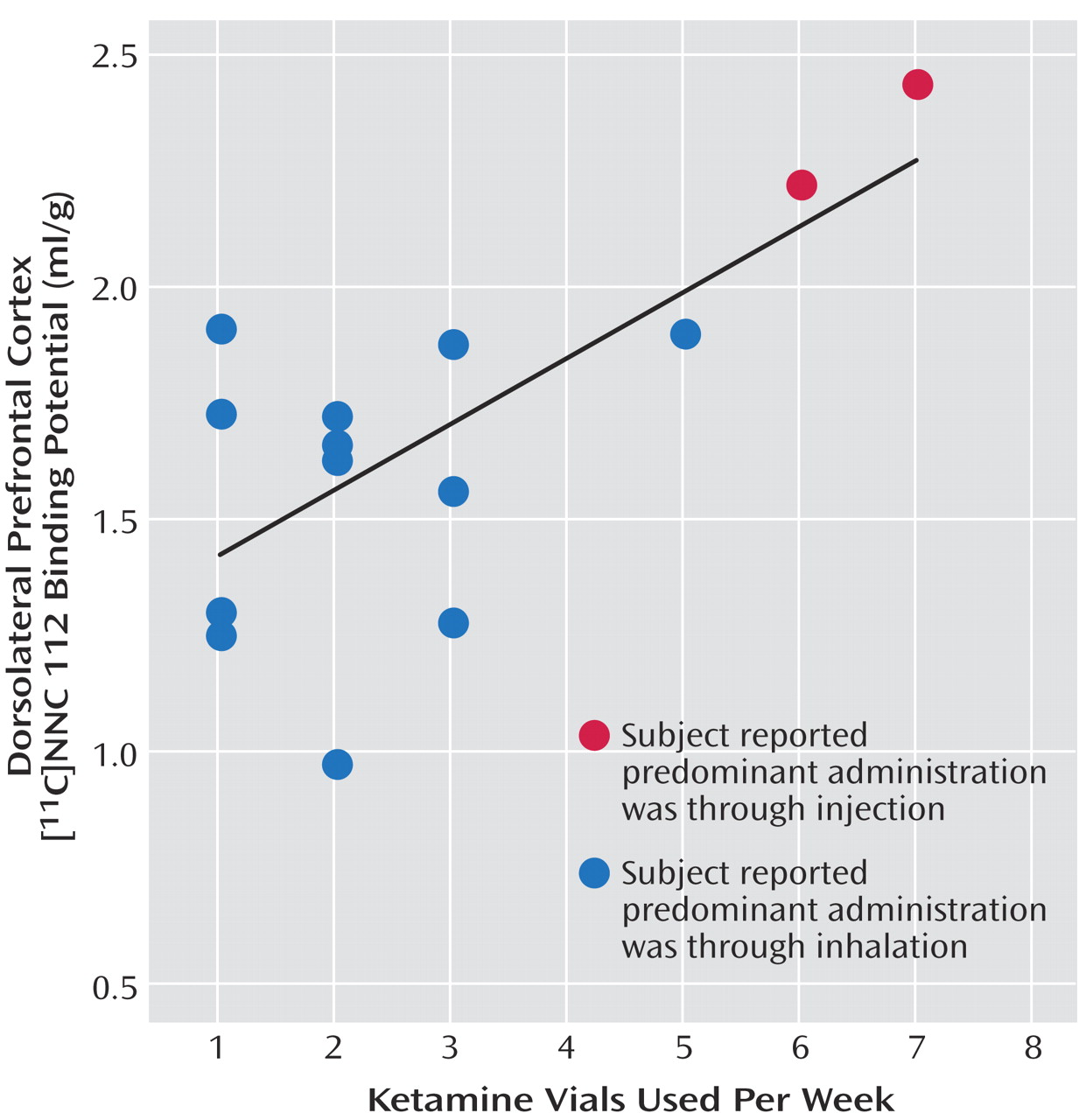
11C]NNC 112 binding potential positively correlated with the number of vials of ketamine used per week (
Figure 2). Dorsolateral prefrontal cortex [
11C]NNC 112 binding potential was not associated with the duration of ketamine use (r
2<0.01, p=0.77) nor with the average hair ketamine concentration (r
2<0.01, p=0.87).
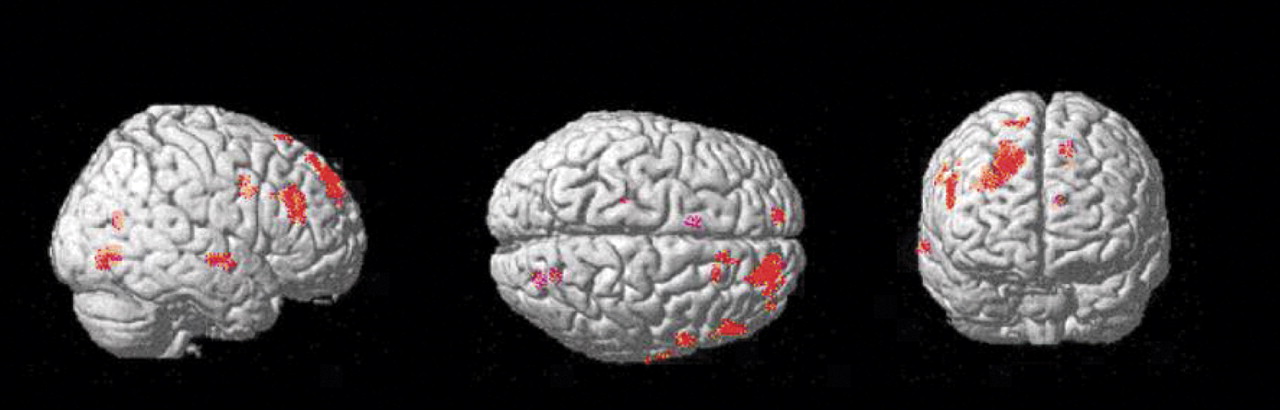
Statistical parametric mapping analysis
Group comparison of [
11C]NNC 112 binding data on a voxel basis (results shown in
Figure 3) revealed various clusters of increased [
11C]NNC 112 in chronic ketamine users relative to comparison subjects. The main area was localized within the dorsolateral prefrontal cortex, confirming the finding of the region of interest analysis. No clusters of decreased [
11C]NNC 112 in chronic ketamine users relative to comparison subjects were identified.
Hair Analysis
Mean hair ketamine concentration in the chronic ketamine users was 32 ng/mg of hair (SD=21, range=9–70). Mean hair concentration was not correlated with the average number of vials reported per week (r2=0.07, p=0.33). This lack of correlation was not unexpected because of possible errors in the reported frequency of use and because of several factors such as hair pigmentation and frequency of hair washing, which affect the relationship between dose exposure and hair concentration.
Neurocognitive Assessment
No significant performance differences were found between the groups on tests involving working memory (semantic, visuospatial, and auditory), executive functions, attention, reaction time, verbal learning and memory, verbal fluency, motor function, and intellectual functioning. Chronic ketamine users performed better than healthy subjects on a social cognition task. In the chronic ketamine users, no significant relationships were found between dorsolateral prefrontal cortex [11C]NNC 112 binding potential and performance on the N-back tests (1-back: r2=0.19, p=0.15; 2-back: r2=0.05, p=0.50; 3-back: r2=0.19, p=0.14).
Discussion
In this study, we investigated D
1 receptor availability in a group of subjects who regularly used ketamine for recreational or mind-exploring purposes. These subjects had a strong and exclusive interest in ketamine (with the exception of two subjects who also abused cannabis). The regular use of ketamine in this cohort did not result in a detectable downward shift in socioeconomic status. As per inclusion criteria, these subjects were free of medical, neurological, and psychiatric conditions while not under the influence of ketamine. In contrast to the ketamine users studied previously, who were polydrug abusers
(12,
13), subjects included in this cohort did not exhibit neurocognitive alterations on a battery of tests administered after 2 days of monitored abstinence. The clinical read and volumetric analysis of the MRI was unremarkable.
In contrast to cognitive performances and regional brain volumes, which were similar in healthy subjects and chronic ketamine users, the PET scan detected an increase in [
11C]NNC 112 binding potential in chronic ketamine users that reached significance only in the dorsolateral prefrontal cortex in both the region-of-interest and voxel-based analyses. The quantitative method used in this study is not sensitive to possible alterations in regional cerebral blood flow that could be associated with ketamine use
(34–
36). In addition, no between-group differences were observed in [
11C]NNC 112 plasma-free fraction, in [
11C]NNC 112 nonspecific binding (cerebellum V
T), or in dorsolateral prefrontal cortex volumes. Under these conditions, increased [
11C]NNC 112 binding potential and V
3′′ clearly indicated increased availability of D
1 receptors. This increased availability could be due to increased receptor density or affinity. The imaging methods used in this study do not discriminate between these mechanisms.
The significance of this finding did not survive correction for multiple comparisons, either in region-of-interest or in statistical parametric analyses. Since animal data indicated that the dorsolateral prefrontal cortex dopamine projections were especially vulnerable to repeated NMDA antagonist administration, this study was primarily designed to look at this region, and under these conditions, no multiple comparison correction is required to ascertain the statistical significance of the findings. Regarding statistical parametric analysis, it has been argued by Friston and colleagues
(31) that the use of “corrected” p values in statistical parametric analysis is unnecessary and inappropriately conservative when the target region of interest is predicted in advance.
It cannot be ascertained if this increased D
1 receptor availability in the dorsolateral prefrontal cortex of chronic ketamine users is associated with a vulnerability to develop ketamine abuse or is a consequence of repeated ketamine exposure. The positive correlation between average number of ketamine vials used per week and dorsolateral prefrontal cortex [
11C]NNC 112 binding potential does not, per se, indicate that this alteration is a result of toxic effects of ketamine. However, the fact that a similar increase in dorsolateral prefrontal cortex [
11C]NNC 112 binding potential has been observed in nonhuman primates chronically treated with the NMDA antagonist MK-801 strongly supports the hypothesis that elevated [
11C]NNC 112 binding potential is a consequence of repeated ketamine exposure. In rodents, glutamatergic projections from the prefrontal cortex to the ventral tegmental area make direct synaptic contacts onto dopaminergic cells that project back to the cortex
(37). This circuit provides an anatomical substrate by which prefrontal dopamine projections might be more vulnerable than other dopamine projections to repeated alterations of glutamatergic transmission induced by ketamine.
The normality of the cognitive performances in the chronic ketamine users was an unanticipated result of this study. From the aforementioned animal studies, it is reasonable to postulate that the D1 receptor up-regulation observed in the present study might be secondary to a drug-induced deficit in prefrontal dopamine function. Given the absence of detectable neurocognitive impairment observed in these subjects, it is tempting to speculate that, at this stage in the condition, the up-regulation of D1 receptors might be relatively efficient at compensating for a deficit in prefrontal dopamine function. However, this up-regulation might be an early sign of system dysregulation. Because of the design of the study, subjects with potentially more severe consequences of NMDA antagonist exposure were less likely to participate, since alcoholism, polysubstance abuse, and comorbid psychopathology tend to develop with the progression of the addiction and were exclusion criteria.
It is very important to stress that the recruitment criteria (absence of psychiatric comorbidity and other substance abuse) used in this study resulted in a sample of “high-functioning” recreational ketamine users, and that this group might not be representative of the majority of ketamine abusers. Therefore, the absence of cognitive deficits in chronic ketamine users enrolled in this study does not indicate that the recreational use of ketamine is safe for cognitive functions. In fact, this study shows that, even in the absence of cognitive deficits, repeated ketamine exposure is associated with signs of disruptions of a critical component of cognition, the prefrontal dopamine system.
The findings of the present study also have implications for the pathophysiology of schizophrenia. Schizophrenia, a severe and chronic mental illness, is believed to be associated with an imbalance in dopamine transmission, characterized by a persistent deficit in prefrontal cortical dopamine function involving D
1 receptors (contributing to the cognitive impairment) and an intermittent excess of subcortical dopamine function involving D
2 receptors (contributing to the emergence of psychotic states)
(38,
39). In addition, several lines of evidence suggest that a deficit in NMDA transmission might be a fundamental aspect of the pathophysiology of this illness
(23–
25). Schizophrenia is associated with a regionally selective up-regulation of [
11C]NNC 112 binding potential in the dorsolateral prefrontal cortex
(22), an observation that might reflect a sustained deficit in presynaptic dopamine function. The fact that chronic ketamine users and patients with schizophrenia exhibit the same endophenotypic trait (up-regulated D
1 receptor expression in the dorsolateral prefrontal cortex) supports the hypothesis that in schizophrenia, this alteration might be secondary to NMDA dysfunction
(40).
In conclusion, repeated exposure to ketamine in humans is associated with up-regulation of D1 receptors in the dorsolateral prefrontal cortex. Animal data suggest that this phenomenon could be related to deficits in prefrontal presynaptic dopamine function induced by intermittent and repeated NMDA blockade. Thus, repeated use of ketamine for recreational purposes might be associated with detrimental effects on brain neurotransmission. Given the critical role of this system in executive functions, more studies are needed to evaluate this neurotoxic effect of ketamine and the reversibility of these changes upon long-term abstinence.