More than 90 years ago, Bleuler
(1, p. 9) proposed the term “schizophrenia” to describe a disease process that produces a “splitting of the psychic functions.” More recently, the failure of a “single-lesion” model to account for the pathophysiology of schizophrenia has led to a renewed focus on “disconnection syndrome” models
(2,
3). These models draw on recent neuroscientific findings and highlight a lack of structural and functional connectivity across multiple brain systems
(4–
6).
Cognitive models of schizophrenia are consistent with disconnection accounts. Common to these models is the proposal that schizophrenia represents a disturbed integration of incoming sensory input with regularities stored in memory
(7–
9). This lack of integration is reflected in deficits of perceptual organization
(10), attention to relevant signals and inhibition of irrelevant signals
(11,
12), and defective metacognitive processes
(13). Braff
(11) has attributed information-processing dysfunctions in schizophrenia to a miscoordination of distributed neural networks that would normally function in an integrated and time-linked manner.
Andreasen and colleagues
(4,
5,
14,
15) coined the term “cognitive dysmetria” to emphasize the temporal dimension of neural disconnection in schizophrenia. It is proposed that a “disruption to fluid coordination of mental activity” at the “nanosecond” time scale impairs the ability to integrate and contextualize incoming sensory input in order to form an appropriate and adaptive response
(15) (p. 911). Andreasen and colleagues hypothesized that a lack of connectivity in cortical-cerebellar-thalamal-cortical circuitry underlies cognitive dysmetria. Although the cortical-cerebellar-thalamal-cortical circuitry spatial dimension of cognitive dysmetria has been investigated
(16–
19), this is the first study to our knowledge to use a high-temporal-resolution measure to investigate distributed brain network integration in first-episode schizophrenia.
Synchronization (phase-locking) has been proposed as a mechanism for the integration, or “binding,” of activity in distributed neural networks
(20,
21). In-phase, or synchronous, neuronal activity has been observed across species and across both microscopic and mesoscopic scales of recording with depth electrode and scalp recordings from EEGs (see reference
22 for a review). Notably, such synchronous activity has been associated with oscillations in the gamma frequency band (varying from 30 to 90 Hz, depending on the task), prompting the description of gamma-band activity as the “building block” of brain function
(23). Synchronous gamma-band activity has been observed in healthy humans in response to sensory stimulation and a range of cognitive tasks
(22), including both auditory and visual stimulation
(24–
26) and learned associations
(27).
Gamma synchrony provides a pertinent index of thalamal-cortical synchrony. In a numerical simulation of whole brain neurophysiology, Rennie et al.
(28) demonstrated that gamma activity is enhanced with increased activity in the thalamal-cortical feedback loop. Jones
(29) proposed that in schizophrenia, a failure of neuronal binding in the cortical-reticulal-thalamal-cortical loop is reflected in disturbed synchronization in the gamma frequency band. This disturbance is associated with an inability to focus attention on relevant stimuli in the face of irrelevant stimuli and to select appropriate responses. To date, studies have mainly focused on abnormalities in the amplitude (or power) of gamma activity in schizophrenia
(30–
33) rather than on the synchronization of gamma activity, which provides a more direct measure of neural binding.
Our group’s study of gamma synchrony in chronic schizophrenia revealed a lack of temporal connectivity across distributed networks, with decreased frontal (gamma 1 and 2) and left hemisphere gamma 1 synchrony but increased posterior gamma 2 synchrony to target stimuli, relative to healthy comparison subjects
(34). These findings are consistent with a core disturbance of temporal connectivity (or “cognitive dysmetria”) across cortical-cerebellar-thalamal-cortical circuitry, which impairs the perceptual and cognitive processing of target stimuli.
The present study investigated gamma synchrony in first-episode schizophrenia. Given that the long-term effects of medication, illness chronicity, and institutionalization are minimized in these individuals, this study provided a first step in elucidating the role of temporal connectivity in the initial stages of schizophrenia
(35,
36). We predicted that the gamma synchrony disturbances observed in chronic schizophrenia
(34) would also be present in first-episode schizophrenia, consistent with the view that disturbances in the temporal connectivity of distributed cortical-cerebellar-thalamal-cortical circuitry are a marker of this disorder.
Discussion
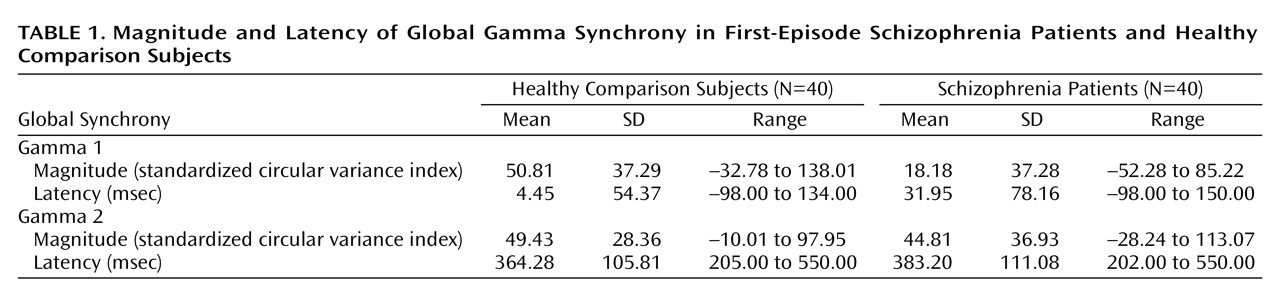
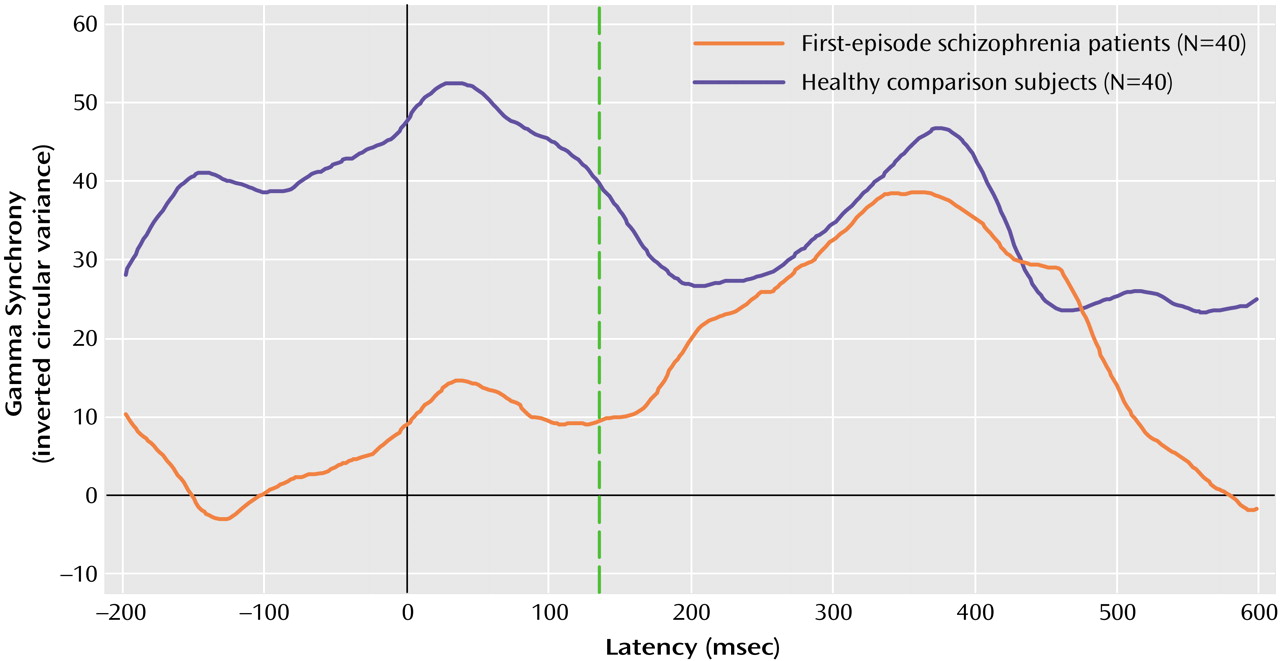
In this study, we used a high-temporal-resolution (millisecond time scale) measure, gamma synchrony, to examine disturbances of functional brain connectivity in first-episode schizophrenia patients versus healthy comparison subjects in response to task-relevant stimuli. The first-episode schizophrenia patients exhibited a decrease in early (gamma 1; –150 to 150 msec poststimulus) synchrony in relation to the healthy comparison subjects. By contrast, there were no impairments in synchrony during the later (200 to 550 msec poststimulus) period of processing. The lower levels of early synchronization of the schizophrenia patients were most apparent in the frontal region. Although the schizophrenia patients showed a gradient of reduced frontal relative to posterior synchrony, the healthy comparison subjects showed the opposite pattern of anterior-posterior synchrony. Although we observed a concomitant delay in early synchrony in the schizophrenia patients, this finding was due, in part, to the effects of neuroleptic medication.
Our findings for first-episode schizophrenia are consistent with models describing schizophrenia as a “disconnection syndrome” in which the precise temporal coordination (or functional connectivity) of distributed neural activity is disrupted
(11,
14,
15). The observation that synchrony was reduced frontally in first-episode patients accords with our group’s previous finding in chronic schizophrenia
(34), as well as preliminary evidence from a study of first-episode schizophrenia in relation to sex differences
(49). The occurrence of this frontal impairment in the early expression of schizophrenia suggests that it is unlikely to be a consequence of chronicity. Rather, a lack of frontal synchronization may have a trait-like status in schizophrenia, suggesting its potential as a marker of this disorder.
The present findings of reduced early frontal synchrony in first-episode schizophrenia are consistent with the “hypofrontality” hypothesis of schizophrenia
(50). Yet these findings suggest that rather than a reduction in volume or activity alone, there is a fundamental lack of functional connectivity in the frontal lobe. This proposal accords with the proposed mechanisms of gamma synchrony. Depth-recording evidence suggests that short-range gamma synchrony results from the mutual inhibition of γ-aminobutyric acid (GABA)-ergic interneurons and long-range synchrony from the precise in-phase synchronization of interneuron networks and pyramidal cells
(51,
52). In postmortem studies, schizophrenia patients showed a selective decrease of 40% in the axon terminal density of chandelier neurons—GABA-ergic interneurons that synapse exclusively with pyramidal cells
(53). The decrease in GABA-ergic binding in schizophrenia is most apparent in the frontal cortex
(54) and is not explained by medication
(53).
The decrease and delay of early gamma synchrony observed in first-episode schizophrenia is also consistent with cognitive models of a perceptual organization deficit in this disorder
(10). This impaired ability to integrate stimulus components into object representations in schizophrenia is believed to occur within 200-msec poststimulus, overlapping the temporal window within which early gamma synchrony occurs. Such a disturbance might lead to an inability to discriminate relevant from irrelevant sensory input into consciousness
(11,
12). Indeed, Clementz et al.
(55) suggested that gamma-band abnormalities might account for schizophrenia reductions in P50 suppression, an index of sensory gating
(11). Our finding that first-episode patients were delayed in identifying target stimuli (albeit accurately) may reflect the flow-on consequences of disruptions to early sensory integration. While reaction time to targets has been associated with gamma 2 synchrony in healthy subjects
(48), the lack of gamma 2 disturbances in first-episode patients suggests that performance delays are not due to a breakdown in more controlled, contextual processing.
Although disturbed frontal connectivity has now been demonstrated in both first-episode and chronic schizophrenia, the stage of processing in which this disturbance occurs appears to differ between the early and chronic phases of this disorder. The lack of temporal connectivity in first-episode patients was associated with the early integration of sensory input only. By contrast, chronically ill patients show desynchronization during later contextual processing (gamma 2) as well as during early sensory integration (gamma 1), which is localized breakdown in the left hemisphere as well as the frontal cortex
(30,
32,
34). Wright and Kydd
(56) proposed the notion of an evolving pathophysiology in schizophrenia, with compensatory maladaptions unfolding in the pursuit of homeostasis. From this perspective, the reduction and delay in early synchrony in first-episode schizophrenia might represent an original state of neural disharmony resulting in sensory overload, with the more topographically distributed breakdown of later context-related synchrony arising as an attempted compensation with illness chronicity. Longitudinal investigation of gamma synchrony in schizophrenia would be important in testing this proposal and in discounting the alternative possibility that chronically ill schizophrenia patients represent a distinct pathophysiology.
Our observation that deficits in synchrony are present in the first episode of schizophrenia and may progress with chronicity is consistent with the notion that schizophrenia may be a “magnification” of variants in connectivity
(57) along a continuum between “normality” and frank psychosis
(58). The investigation of gamma synchrony in high-risk individuals might provide an understanding of the links between schizophrenia and normality, as well as earlier stages in the development of schizophrenia
(11).
Peled
(59) has suggested that different patterns of psychopathology within schizophrenia might emerge from different patterns of failed neural connectivity (or “multiple constraint organization”). Our group
(34) found evidence of different patterns of gamma synchrony disturbances associated with psychomotor poverty, reality distortion, and disorganization syndromes in chronic schizophrenia. The investigation of relationships between symptom profiles and gamma synchrony disturbances in a first-episode sample might provide further understanding of the clinical implications of gamma synchrony disturbances.
Future studies are also warranted to clarify several other matters regarding disturbances of neural synchronization in first-episode schizophrenia. First, although medication dose did not account for disturbances in the magnitude of gamma synchrony in this study, the correlational design makes it difficult to explicitly disentangle the potential contribution of medication. Therefore, it would be important to confirm these findings of impaired synchronization in a direct comparison of medicated and unmedicated first-episode schizophrenia patients. Second, the employment of a variety of cognitive paradigms would also help to elucidate the relationship between gamma synchrony abnormalities and dysfunctions of sensory and information processing. It is possible that more complex tasks—and a greater cognitive load—would elicit gamma 2 as well as gamma 1 synchrony disturbances in first-episode schizophrenia patients. In extending this research to other cognitive tasks, the contribution of synchronization in other frequency bands and its functional significance should also be considered
(60). Third, the relationship between disturbed gamma synchrony and constitutional characteristics such as gender warrants attention, given the gender bias in the incidence of schizophrenia and preliminary indications that gamma synchrony varies with gender
(49). Fourth, it would be important to examine the specificity of these disturbances to the diagnosis of schizophrenia, particularly in relation to other psychotic disorders, given overlaps in symptom profiles
(35,
61) and the nonspecificity of neuroleptic medications for psychotic symptoms
(62). A comparison of schizophrenia and affective psychosis is currently underway.
The present study is the first to our knowledge to demonstrate disturbed temporal connectivity in first-episode schizophrenia with a gamma synchrony measure. The reduction and delay in early synchrony in the frontal cortex might represent a marker for the early manifestation of schizophrenia. The lower level of early frontal synchrony might represent a trait-like marker of schizophrenia that is consistent with neurobiological evidence of selective GABA-ergic deficits in the frontal cortex as well as cognitive evidence for an impairment in perceptual integration. Given that GABA-ergic mechanisms have been implicated in the action of antipsychotic medications and the generation of gamma synchrony, an understanding of gamma activity has the potential to provide a basis for improved interventions for schizophrenia.