Studies of sensitivity to the bitter-tasting antithyroid compound phenylthiocarbamide (PTC) have shown this to be an inherited trait determined by a dominant allele
(1,
2). A G protein-coupled bitter taste receptor, TAS2R38 (also known as PTC or TAS2R), located on chromosome 7q, accounts for 85% of the variance in taste
(3). A second minor locus is found at 16p
(4). About 30% of the U.S. population are PTC “nontasters” (i.e., tt), while approximately 70% are “tasters” (i.e., TT or Tt)
(4,
5). Although nontaster status has been linked to a variety of medical disorders, there have been few investigations of PTC taster status in schizophrenia
(6–
8). Abnormalities in the function and/or expression of G protein signaling pathways have been reported in patients with schizophrenia and appear to be implicated in prepulse inhibition of the startle reflex and negative symptoms
(9,
10). Therefore, we anticipated that patients would show a differential pattern of PTC tasting status relative to healthy comparison subjects and that a similar pattern would be observed in their non-ill first-degree relatives.
Method
Forty-two patients with schizophrenia (30 men and 12 women), 23 healthy volunteers (11 men and 12 women), and 12 first-degree relatives (four men and eight women) were recruited from the University of Pennsylvania Schizophrenia Research Center. The relative cohort included one parent, eight siblings, and three adult offspring of six patients. The patients received the Structured Clinical Interview for DSM-IV, Patient Edition
(11), a physical examination, and routine laboratory tests. The patients were rated on the Brief Psychiatric Rating Scale (BPRS)
(12), the Scale for the Assessment of Negative Symptoms
(13), and the Scale for the Assessment of Positive Symptoms
(14). Family members were assessed with the Structured Clinical Interview for Personality Disorders
(15). All probands met DSM-IV criteria for schizophrenia with no other concurrent diagnoses. Family members and healthy comparison subjects were free of any current axis I diagnosis or axis II cluster A personality disorder.
Subjects were excluded for a history of neurological disorder, head trauma, loss of consciousness, substance abuse/dependence, a medical condition that might alter cerebral functioning, a recent respiratory infection, or any condition that could affect taste functioning. Written informed consent was obtained after a complete description of the study was given to the subjects.
There were no differences in age among the patients (mean=36.7, SD=12.7), family members (mean=41.2, SD=18.8), and healthy comparison subjects (mean=30.8, SD=12.8) (F=2.5, df=2, 74, p=0.09). The probands had a greater proportion of African Americans than the healthy comparison subjects (χ2=12.8, df=6, p<0.05). All patients were stable outpatients at the time of testing. Mean duration of illness was 11.8 years (SD=8.6). Twenty-five patients were receiving atypical antipsychotic medications, six were receiving typical antipsychotics, and 11 were unmedicated at the time of testing. The mean dose was 323.3 mg/day (SD=213.6) in chlorpromazine equivalents. The mean BPRS score was 30.4 (SD=8.6), indicating a low level of acute symptoms.
A PTC-impregnated strip of filter paper (Carolina Biological Supply Company, Burlington, N.C.) was placed on the tongue. The subjects were asked if they tasted anything. They were then asked to rate the intensity on a 100-mm visual analog line, ranging from 0 mm (no taste) to 100 mm (extremely strong taste). Any subject who reported an inability to taste the filter paper and an intensity of less than 6 mm was classified as a nontaster. The 6-mm value denoted the break point in the bimodal distribution of intensity ratings between the tasters and nontasters.
Results
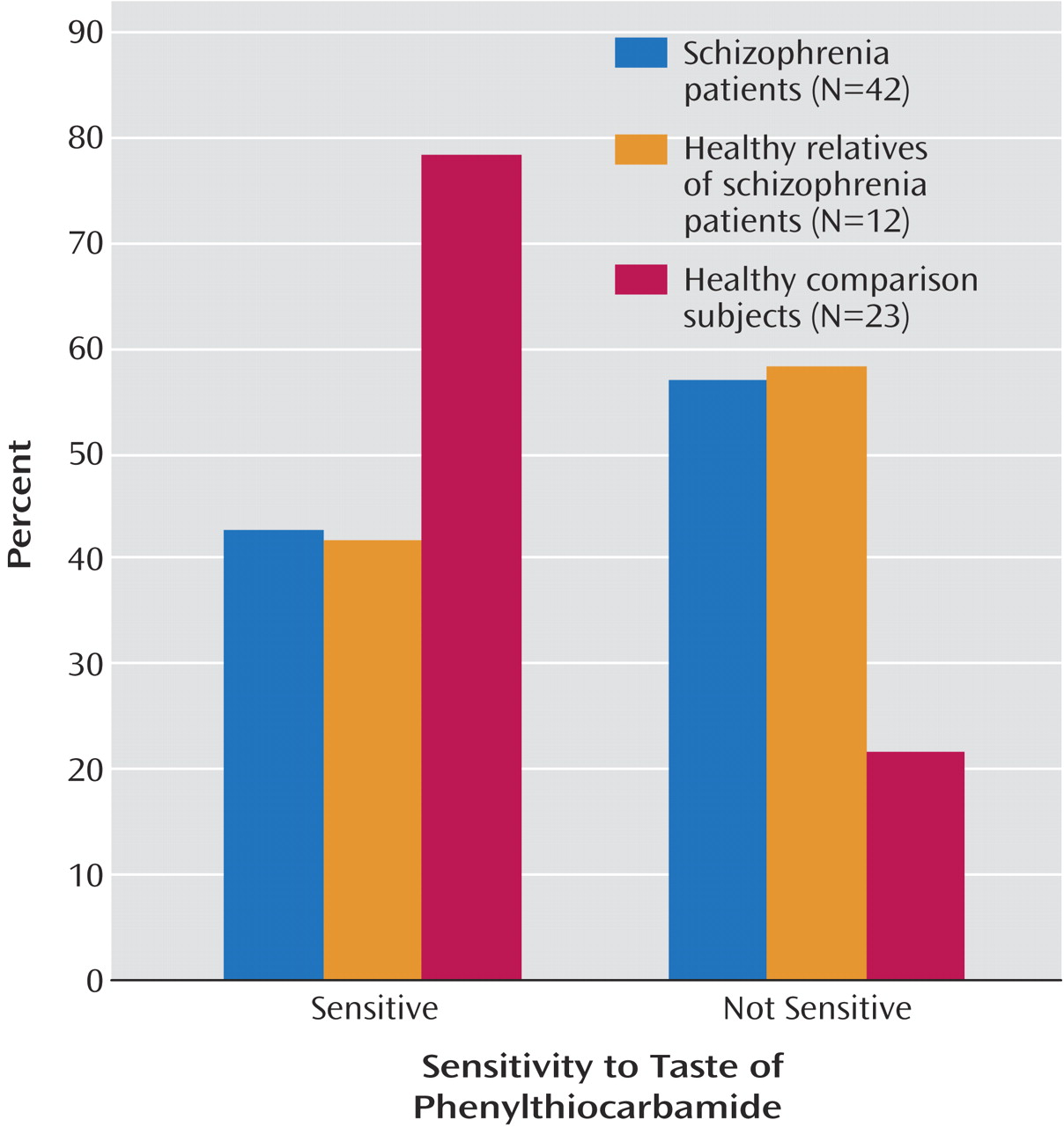
Statistical analysis revealed significant differences in the distribution of tasters and nontasters among the three groups (χ
2=8.30, df=2, p<0.02), with both patients (χ
2=7.53, df=1, p=0.006) and family members (χ
2=4.70, df=1, p=0.03) having a larger proportion of nontasters than healthy volunteers (
Figure 1). Patients and family members did not differ from one another (χ
2=0.01, df=1, p=0.94). Among tasters, there were no group differences in intensity ratings and no effect of diagnosis, sex, or diagnosis-by-sex interaction (all p>0.18).
Within the patient group, tasters and nontasters did not differ in sex distribution, Mini-Mental State Examination (MMSE) score, age at onset, illness duration, illness severity, deficit status, or negative symptoms. The two subgroups did differ in positive symptom ratings (F=5.3, df=1, 33, p<0.03), with nontasters demonstrating higher total scores on the Scale for the Assessment of Positive Symptoms. This reflected higher ratings for the hallucinations (F=7.6, df=1, 33, p=0.009) and delusions (F=5.3, df=1, 33, p<0.03) subscales. Linear regression analyses with age, sex, chlorpromazine equivalents, age at onset, illness duration, smoking history, and MMSE score as predictors did not alter the observed differences in taster status or affect PTC intensity ratings.
Discussion
These data demonstrate a higher prevalence of PTC nontasters among patients with schizophrenia and their non-ill first-degree relatives. Among healthy comparison subjects, 78% were classified as tasters, in contrast to 43% among patients and 42% among family members. The higher prevalence of nontasters in the patient and family groups is consistent with three other reports of nontaster prevalences ranging from 42% to 47% among patients with schizophrenia
(6–
8). A higher prevalence of nontasters in patients could not be explained by a greater proportion of African Americans because this ethnic group shows a lower prevalence of nontasters than other populations
(4). The presence of a similar impairment in first-degree relatives suggests that people with at least one dominant allele may be at a lower risk than those with two recessive alleles. The high frequency of nontasters in patients and family members may be due to abnormalities in the function and/or expression of G-protein signaling pathways. Drayna and colleagues
(16) found that PTC binds to both forms of the receptor (i.e., taster and nontaster) with equal affinity, but the nontaster form fails to activate G-protein. This failure in G-protein signaling may interact with other genetic and/or environmental factors to produce an increased vulnerability to illness. This hypothesis requires further investigation.
A few caveats must be noted. First, the group of family members was relatively small, and a larger cohort will be required to fully assess this difference. However, it is notable that the effect size (Cohen’s d) for the difference in taster status between family members and healthy comparison subjects is quite large (d
+=0.81, 95% confidence interval [CI]=0.08–1.53), and we would expect this effect to be robust in independent samples. Second, the comparison subject group was smaller than the group of patients. Nevertheless, the observed proportions of tasters and nontasters in the healthy group were consistent with other population-based investigations of PTC sensitivity
(4).
We conclude that PTC nontaster status may be an endophenotypic marker of an inherited neuronal abnormality that conveys risk for the development of schizophrenia.