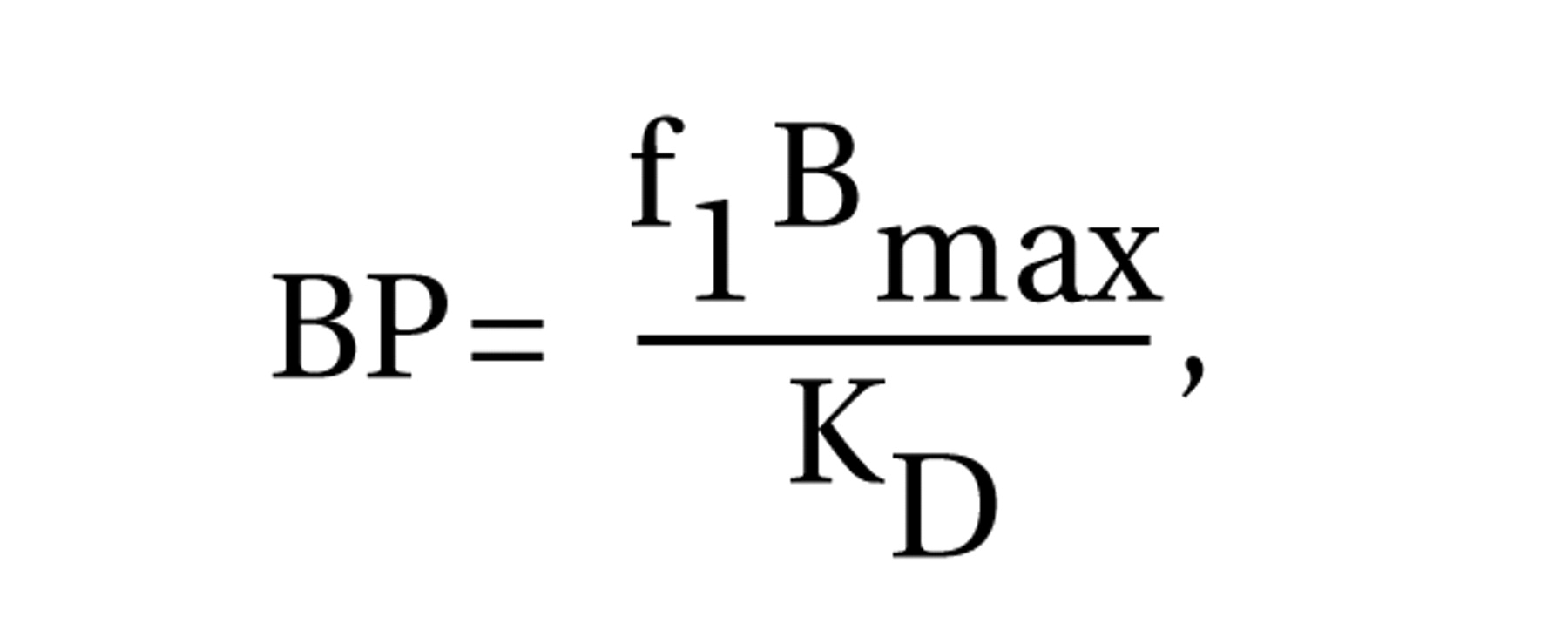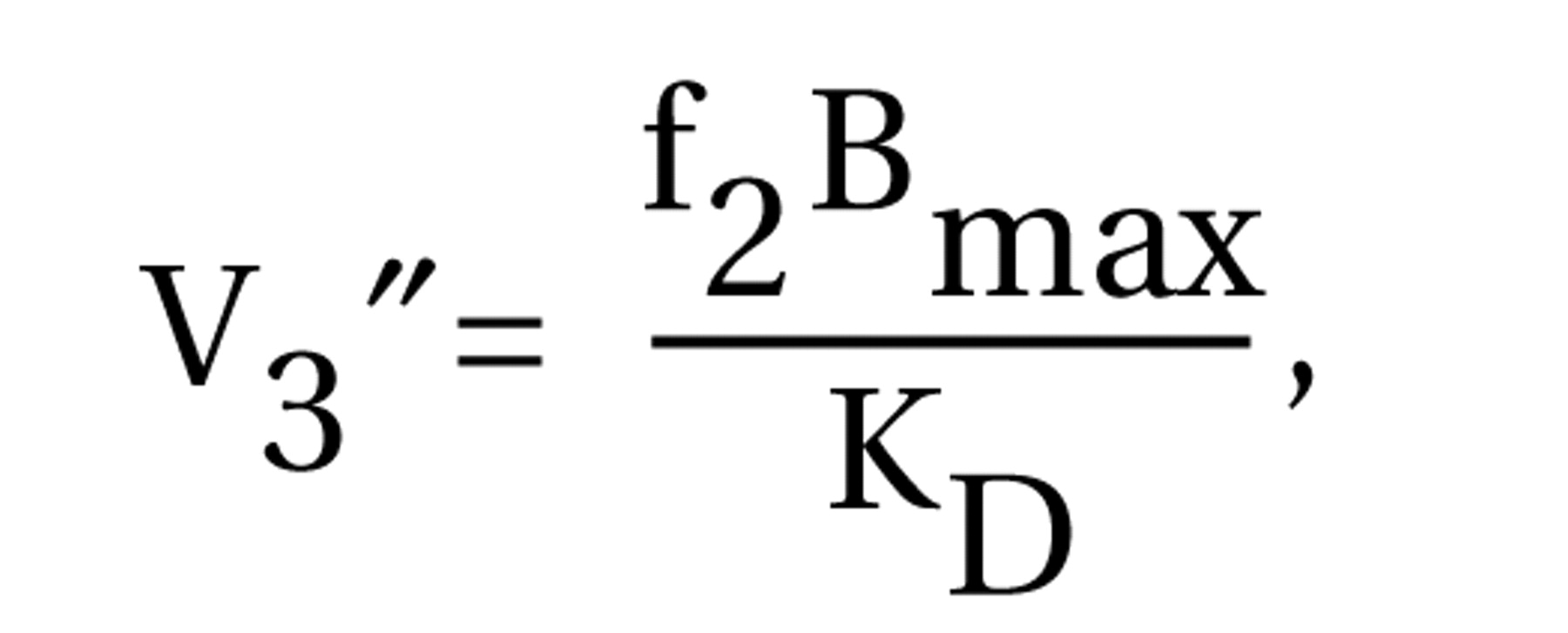Reduced activity of the serotonin (5-HT) system has been implicated in impulsive violence and aggression in studies that have used a variety of paradigms, including measurement of CSF serotonin metabolites, hormonal response to serotonergic probes, and imaging metabolic changes with serotonergic agents. In initial studies, reduced CSF concentration of the serotonin metabolite 5-hydroxyindoleacetic acid was demonstrated in individuals with a history of aggression
(1,
2). Subsequent studies have linked this finding to impulsive aggression
(3). Moreover, a blunted hormonal response to pharmacological manipulation of central serotonin function has been observed in personality disorder patients with impulsive aggression
(4,
5).
Studies of brain lesions have pointed to the orbitofrontal cortex and the anterior cingulate gyrus as key areas regulating the generation of aggressive behaviors
(6–
10). Irritability and angry outbursts have been associated with orbitofrontal cortex damage in neurologic patients
(11). Lesions of the medial orbital cortex early in childhood can result in antisocial, disinhibited, aggressive behavior later in life
(12). These studies suggest that the orbital frontal and adjacent medial frontal cortex exert an inhibitory influence on aggressivity. Studies combining positron emission tomography (PET) imaging of regional glucose metabolism with pharmacologic challenges aimed at increasing serotonergic function also point toward altered 5-HT function in these regions. In healthy subjects, a single dose of the serotonin-releasing agent fenfluramine resulted in an increase in glucose metabolism in the orbitofrontal cortex and medial frontal and cingulate regions. Such an increase was not observed in subjects with impulsive aggression
(13). Furthermore, patients with impulsive aggression demonstrated altered metabolic response to the serotonergic agent meta-chlorophenylpiperazine (
m-CPP) in the orbitofrontal cortex and the anterior cingulate cortex
(14). Together, these findings are consistent with the existence of reduced 5-HT function in the orbitofrontal cortex and anterior cingulate cortex in subjects with impulsive aggression.
In this study, we assess regional brain serotonin innervation in subjects with impulsive aggression by using in vivo imaging of serotonin transporter with PET and [
11C](+)-6β-(4-methylthiophenyl)-1,2,3,5,6α,10β-hexahydropyrrolo[2,1-a]isoquinoline ([
11C]McN 5652). This radiotracer has been successfully developed to image serotonin transporter density in humans
(15–
19) and has been used in a number of clinical studies, including studies of patients with mood disorders
(20), obsessive-compulsive disorder
(21), and ecstasy abuse
(22,
23). Given that the strongest finding in our
m-CPP study was reduced activation of the cingulate cortex
(14), the main hypothesis of this study was that individuals with impulsive aggression would have reduced serotonin transporter density in the anterior cingulate cortex. Serotonin transporter density in the orbitofrontal cortex is too low to be accurately measured with PET and [
11C]McN 5652
(18), so this region was not evaluated in this study.
Discussion
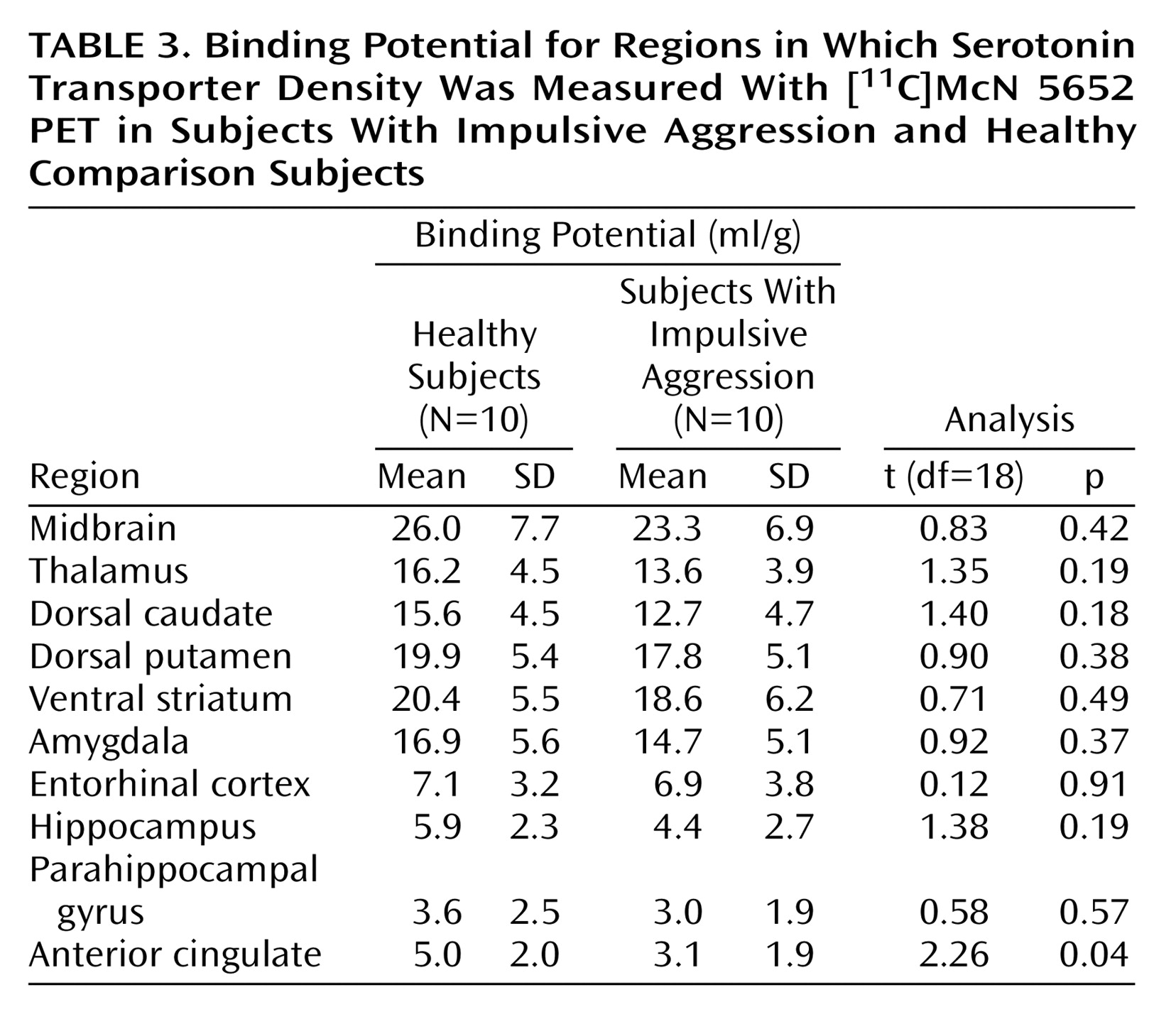
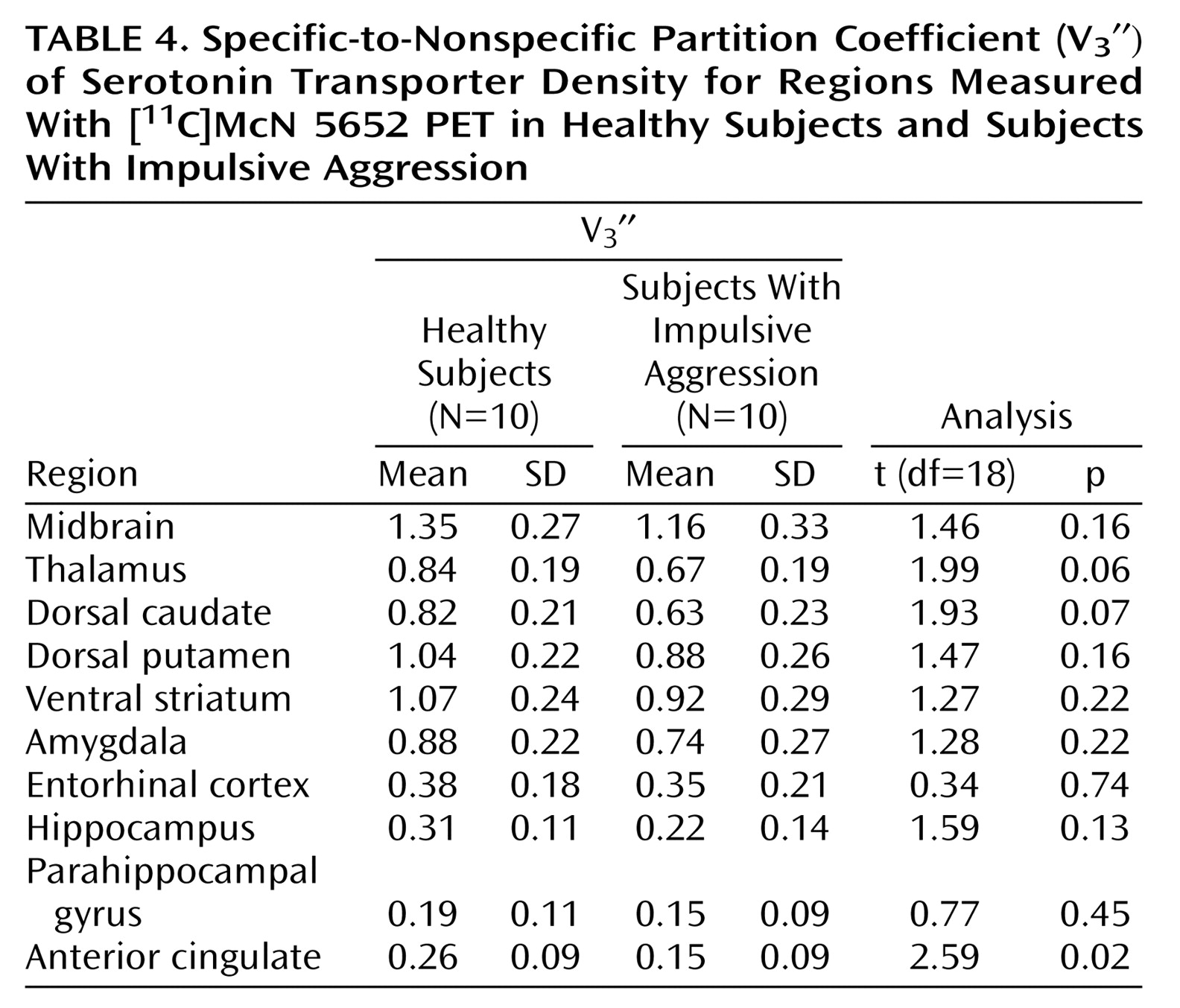
This study suggests that pathological impulsive aggressivity is associated with a reduction in serotonin transporter availability in the anterior cingulate cortex, a reduction that might reflect reduced 5-HT innervation. Although not statistically significant, our data also suggest a modest decrease in serotonin transporter availability in other regions.
The use of a fully quantitative imaging method was a strength of this study. The kinetic analysis is not affected by potential group differences in radiotracer plasma clearance or regional cerebral blood flow
(50). Measurement of the arterial input function enabled the quantitative derivation of distribution volumes, from which both binding potential and V
3″ were calculated. Although methods for deriving [
11C]McN 5652 V
3″ without arterial sampling have been proposed
(20), only with the arterial input function could we demonstrate the absence of group differences in cerebellum distribution volumes and thus validate the use of V
3″ for between-group comparisons of receptor parameters. In the absence of group differences in cerebellum distribution volumes, results derived with binding potential and V
3″ (i.e., binding potential normalized by the nonspecific distribution volume) are essentially similar, which was the case here.
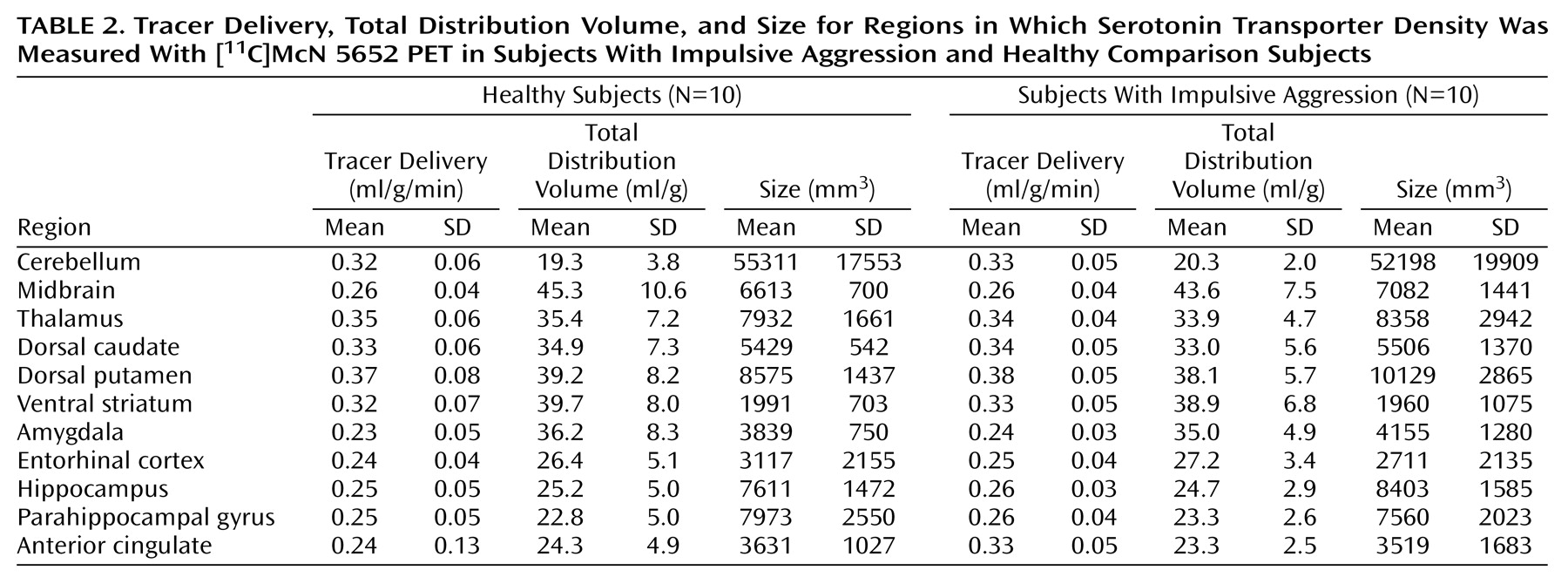
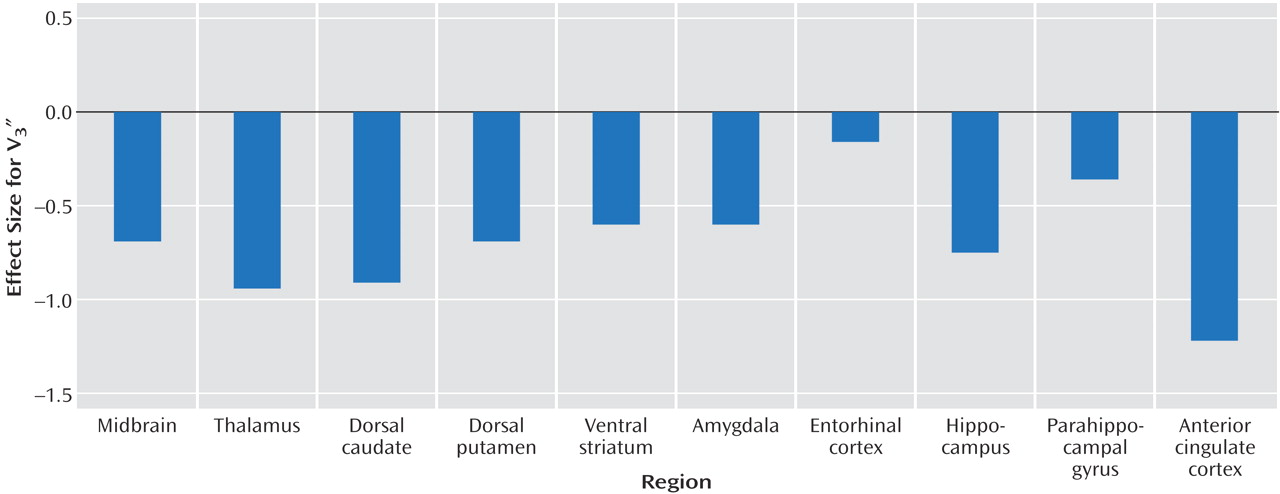
This study has several limitations. The small size of this study group was adequate to detect the relatively large decrease in serotonin transporter in the anterior cingulate cortex in subjects with impulsive aggression but limited our power to detect potential significant differences in other regions. As described, in all regions, V3″ values were lower for the subjects with impulsive aggression than the healthy subjects. Analysis of a larger group would be required to further explore this difference. Examination of the overall effect size for the repeated-measures ANOVA reveals that approximately 30 individuals per group would be required to detect this difference at the level of p<0.05. The effect size (d) for the differences in the individual regions ranged from 1.22 in the anterior cingulate cortex to 0.16 in the entorhinal cortex.
Another limitation of this study arises from the use of [
11C]McN 5652 to measure serotonin transporter. This radiotracer is associated with high levels of nonspecific binding, limiting the ability to quantify serotonin transporter in regions of low serotonin transporter density such as the neocortex
(18). The ability to detect differences in these regions will likely be enhanced by the use of newly developed radiotracers for serotonin transporter such as [
11C]3-amino-4-[2-[(dimethylamino)methyl]phenylthio) benzonitrile ([
11C]DASB)
(51) and [
11C]2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine ([
11C]AFM)
(52,
53). Both tracers markedly improve the signal-to-noise ratio compared with [
11C]McN 5652
(29,
53).
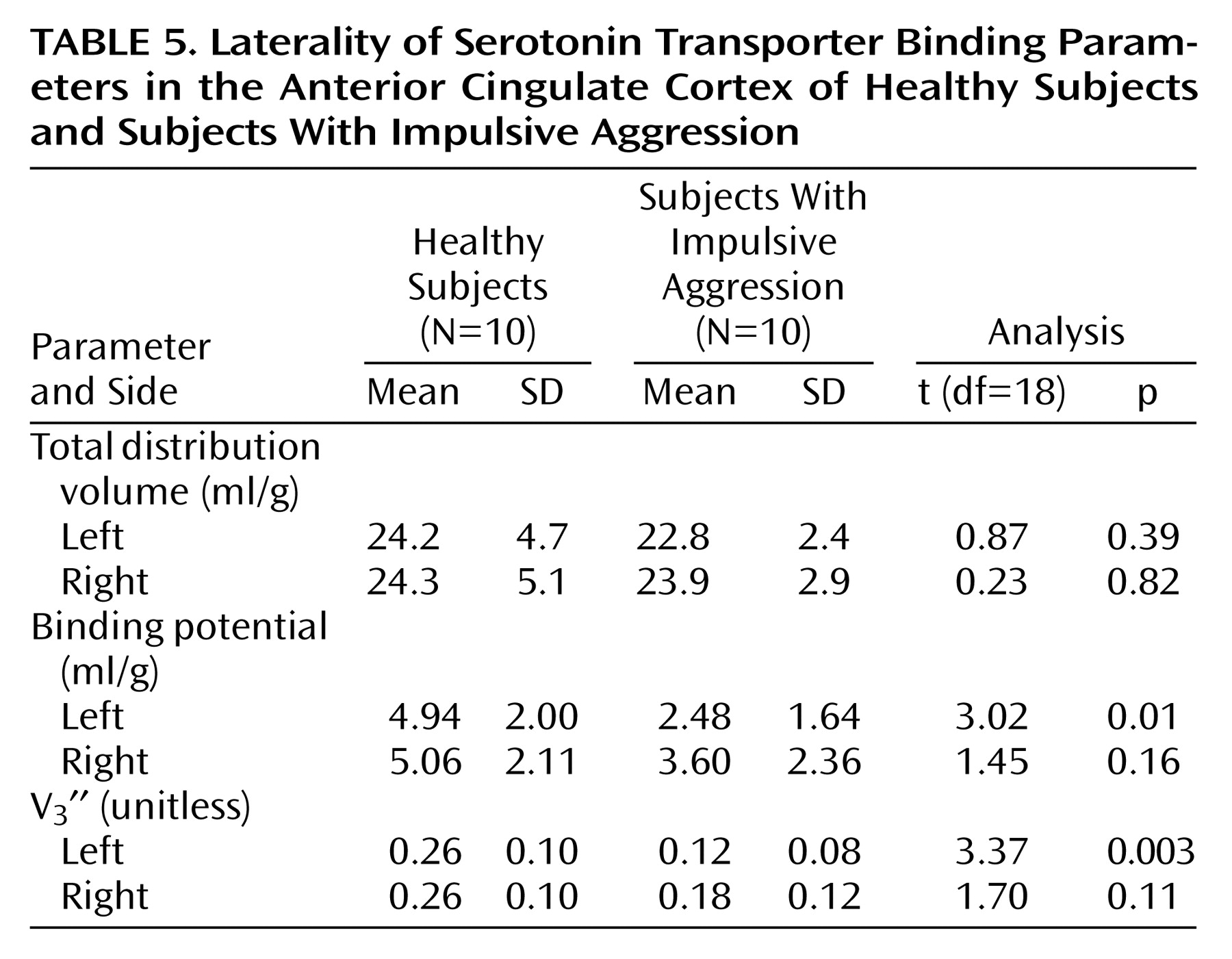
Given previous studies implicating the anterior cingulate cortex in impulsive aggression, this area was the primary focus of our study. The finding of a reduction in serotonin transporter density in this region is consistent with results from studies of cerebral metabolism in impulsive aggression. In response to serotonergic challenges, including d,l-fenfluramine and
m-CPP, the relative glucose metabolic rate of individuals with impulsive aggression is blunted in the anterior cingulate cortex relative to healthy subjects
(13,
14). Fenfluramine acts by causing a direct release of serotonin and antagonizing its reuptake, whereas
m-CPP acts as a partial agonist at the postsynaptic 5-HT
2A and 5-HT
2C receptors. On the basis of these mechanisms of action alone it is not possible to comment on the locus of the abnormality in impulsive aggression, i.e., whether the blunted response is secondary to a pre- or postsynaptic problem. Our study provides some insight into this question, suggesting that a presynaptic deficiency in serotonin innervation exists in the anterior cingulate cortex in this disorder. This result is in line with the observations from studies of serotonin metabolites that have linked reduced serotonin markers with impulsive aggression
(1,
3,
54). Our findings are also in agreement with the postmortem finding of a decrease in serotonin transporter binding in suicide victims
(55), viewed as a specific form of self-directed impulsive/aggressive behavior, as well as the finding of reduced platelet serotonin transporter in aggression
(56).
Davidson et al.
(57) proposed that disruption of the normal regulation of emotion–which involves several interconnected brain regions including the orbitofrontal cortex, amygdala, and anterior cingulate cortex—plays a role in the generation of violence. The anterior cingulate cortex can be separated into a dorsal “cognitive” portion and a rostral-ventral “affective” region
(58). Evidence from a variety of domains indicates that the affective subdivision of the anterior cingulate cortex regulates the intensity of response to emotional stimuli. For example, stimulation of this area in animal models increases the latency of attack behavior
(59). In humans, PET studies of cerebral blood flow demonstrate activation of the ventral anterior cingulate cortex when anger is induced in healthy men using imagery
(60). This same area is activated when symptoms are provoked in individuals with simple phobia, OCD, or PTSD
(61–
63). Further evidence that the rostral-ventral anterior cingulate cortex plays a role in emotional regulation comes from the finding that this area was activated when men attempted to suppress sexual arousal in response to erotic film excerpts but not in the nonsuppression condition
(64). Given the aforementioned limitations of the radiotracer, we were unable to separate the anterior cingulate cortex into the dorsal and rostral-ventral components. However, we did examine the laterality of our finding. Previously, there have been no reports of aggression-related laterality in the anterior cingulate cortex, although work by our group has demonstrated blunted metabolic response to
m-CPP in the left orbitofrontal cortex
(14). The results of this study, indicating a left-sided predominance of the abnormality, are consistent with findings showing that traumatic brain lesions to the left frontal cortex give rise to aggression and hostility, whereas right-sided lesions lead to anxiety/depression
(8).
Our finding of an abnormality of the serotonergic innervation in the anterior cingulate cortex in subjects with impulsive aggression is consistent with the hypothesis that alterations in the normal function of this area may lead to difficulties with affect and impulse modulation, resulting in increased impulsivity and aggression.