Functional imaging studies during mania have been limited, no doubt because of difficulties in having manic patients remain still during scanning. Activation studies published to date have primarily assessed prefrontal cortex reactivity
(1–
3). As enlarged amygdala
(4–
6) and heightened activation
(7) have been reported in some subjects with bipolar disorder, we used a task known to activate the amygdala in normal subjects
(8,
9) to assess amygdala reactivity and frontal-amygdala circuitry during mania.
Method
The study was approved by the institutional review boards of UCLA and the VA Greater Los Angeles Healthcare System. Nine manic subjects with bipolar I disorder (six women and three men; mean age=34.6 years, SD=8.0) diagnosed according to the Structured Clinical Interview for DSM-IV and nine healthy comparison subjects (six women and three men; mean age=30.4 years, SD=7.6) gave written informed consent. The manic subjects had been ill on average for 14.8 years (SD=5.1) and had on average 4.2 (SD=2.0) previous episodes of mania, a mean score on the Young Mania Rating Scale of 15.1 (SD=3.7), and no other axis I comorbidity. Exclusion criteria were left-handedness, hypertension, neurologic illness, metal implants, history of skull fracture, or head trauma with loss of consciousness longer than 5 minutes. Two manic subjects were not receiving medications at the time of scanning; seven had recently initiated treatment with one or more of the following: divalproex sodium (N=4), lithium (N=2), gabapentin (N=2), and olanzapine (N=1).
MRI scans were obtained on a 3-T instrument (General Electric, Waukesha, Wis.) with echo planar imaging capability (Advanced NMR Systems, Wilmington, Mass.). Coplanar single-point, high-resolution structural images (TR/TE=4,000/54 msec, 26 slices, thickness=4 mm, gap=1 mm, matrix=128×128, field of view=20 cm) were obtained. From these, slices for fMRI scanning were chosen covering an area that started below the amygdala and ran superior to Brodmann’s areas 46/9, which were superimposed on the structural images. Functional MRI scanning was conducted with an asymmetrical spin echo acquisition sequence (TR/TE/180° pulse offset=2500/70/25 msec, 16 slices, thickness=4 mm, gap=1 mm, matrix 64×64, field of view=20 cm).
The matching paradigm consisted of three different experimental conditions (“match affect,” “identify affect,” and “match forms”) and included nine experimental blocks: four blocks presented faces and five presented geometric forms. Each block lasted 32.5 seconds for a total scan length of 4:53 minutes
(9). In some blocks, subjects matched a face with one of two other affectively charged faces (“match affect”). In other blocks, subjects identified one of two words that best matched an affectively charged face (“identify affect”). Subjects also performed a control task in which they matched an elliptical form to one of two other forms (“match forms”). As the match affect task condition robustly activates the amygdala, we report here results on this condition. The order of task presentation was balanced in the manic and healthy subjects. During imaging, subjects responded by pressing one of two buttons with their right hand.
Data were corrected for head motion and realigned to a site-specific atlas using automated image registration
(10). Data were smoothed using a 6-mm full width at half maximum Gaussian kernal. The group data were processed statistically using SPM 99 (http://www.fil.ion.ucl.ac.uk/spm/). Fixed effects within-group analyses compared the match affect (experimental) task versus the match forms (control) task. For between-group analyses, we performed an a priori amygdala region of interest analysis. Amygdala regions of interest were drawn on unnormalized scans in each subject’s native space. All voxels exceeding a T threshold of 1.6 (corresponding to p<0.05) were identified using locally developed software. Percent change was then calculated using change from the mean intensity of the control condition (match forms task) based on raw signal intensity values normalized for each subject. A mixed model approach was used (PROC mixed SAS, Version 8.1, SAS Institute, Inc., Cary, N.C.), with the dependent variable being the mean percentage change in MR signal intensity.
Results
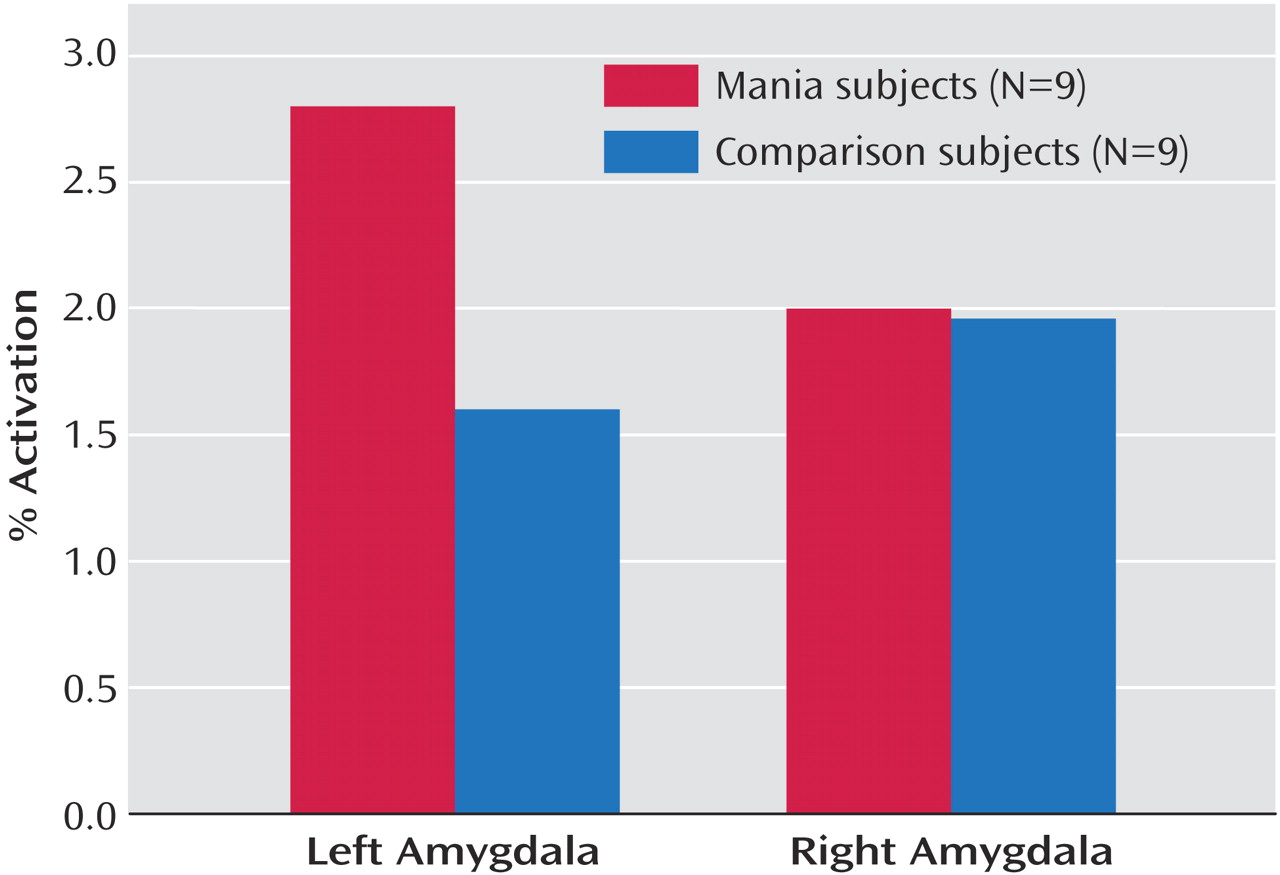
No significant between-group differences were seen for response time (t=0.18, p=0.25) or accuracy (t=0.69, p=0.50) during performance of the task. Region of interest analyses revealed a significant group-by-hemisphere interaction in amygdala activation (F=7.91, df=1, 806, p=0.005). Manic subjects showed a significantly greater percent change in MRI signal activation in the left amygdala relative to age- and gender-matched comparison subjects (t=2.03, df=806, p=0.04) (
Figure 1). No significant differences were observed for the right amygdala (t=0.00, df=806, p=0.96). Statistical parametric mapping analyses (with extent threshold [k]=0; height threshold=4.77; and corrected p=0.05 for both groups) additionally revealed an area of significant bilateral activation during the match affect task in healthy subjects in the lateral orbital prefrontal cortex (Brodmann’s area 47) (Talairach coordinates (x, y, z)=46, 26, –4 [k=31, z score >8, corrected p<0.0001]; and –50, 20, –4 [k=11, z score=6, corrected p<0.0001]); no such activation was observed in the manic subjects.
Discussion
Our results suggest heightened amygdala activation and perhaps reduced statistical parametric mapping activation of orbitofrontal cortical activity during mania relative to that seen in the healthy comparison subjects. The amygdala plays a critical role in modulating social/emotional behavior
(11,
12), both of which are impaired in mania. Amygdala stimulation in animals has been associated with increased dopamine in the nucleus accumbens and in motor centers, resulting in increased motor activity
(13). In humans, activation may result in some clinical correlates of mania.
Whether amygdala activation represents a primary pathophysiologic source in mania or represents a change occurring secondary to a change elsewhere in the brain (e.g., the orbitofrontal cortex) remains to be elucidated. The number of subjects was too small to allow between-group comparisons of the orbitofrontal cortex using statistical parametric mapping. However, our results are consistent with other activation studies demonstrating reduced prefrontal activation in five
(1), six
(3), and 11
(2) subjects with mania. The amygdala receives inputs from the orbitofrontal cortex, temporal lobe association cortex, and multiple subcortical regions
(13). Reduced activity in a brain region such as the orbitofrontal cortex that might normally exert an inhibitory/modulatory effect on the amygdala could result in disruption of a primarily inhibitory prefrontal-amygdala circuit.
In our study, most subjects were receiving treatment, even if just recently started. Future studies with manic subjects receiving no medication may further elucidate the relationship between amygdala function, orbitofrontal function, and mood state.