In this article, we briefly review the epidemiology of nicotine dependence, the efficacy of nicotine dependence pharmacotherapies, and the pharmacogenetics of nicotine dependence treatment. Nicotine dependence is a complex trait, the end result of genetic susceptibility interacting with environmental risk factors to produce the syndrome
(1). Similarly, response to pharmacotherapy in nicotine dependence is a complex trait. Pharmacotherapy for nicotine dependence is important to psychiatrists, as epidemiological studies indicate that a majority of individuals with schizophrenia or affective disorders are daily smokers
(2).
Epidemiology of Nicotine Dependence
Cigarette smoking is implicated in approximately 400,000 deaths annually
(3). Cigarette smoking is the greatest preventable cause of cancer, accounting for at least 30% of all cancer deaths
(4). Approximately 23% of American adults are cigarette smokers
(5). The nicotine in tobacco is the primary rewarding compound that establishes and maintains tobacco use
(5,
6), and most persons who smoke regularly (daily for at least 1 month) develop nicotine dependence
(6–
8). Regular smoking typically begins in adolescence
(5,
9–11).
A complex set of factors allows for the initiation into smoking; those factors include the smoking status of friends and parents, economic status, and heritable factors
(7,
12). Factors that facilitate continuation of smoking probably involve a complex interaction between aversive and rewarding influences of nicotine, as well as environmental variables, including peer group approval and economics. Continued regular smoking leads to nicotine dependence
(7). For a DSM-IV diagnosis of nicotine dependence, a smoker must meet three or more of the following six major criteria:
1.
Tolerance (e.g., the absence of nausea or dizziness despite substantial levels of smoking)
2.
Withdrawal
A.
Daily use for at least several weeks
B.
After abrupt cessation, reports of four or more of the following signs:
•
(1) dysphoric or depressed mood
•
(3) irritability, difficulty managing anger
•
(5) difficulty in concentration
•
(8) increased appetite or weight gain
3.
Repeated unsuccessful attempts to quit or reduce nicotine use
4.
Reduction or elimination of social or occupational activities because smoking tobacco is not allowed in those settings
5.
Continued use despite medical or psychological harm
6.
Use that is often greater than intended or more frequent than intended
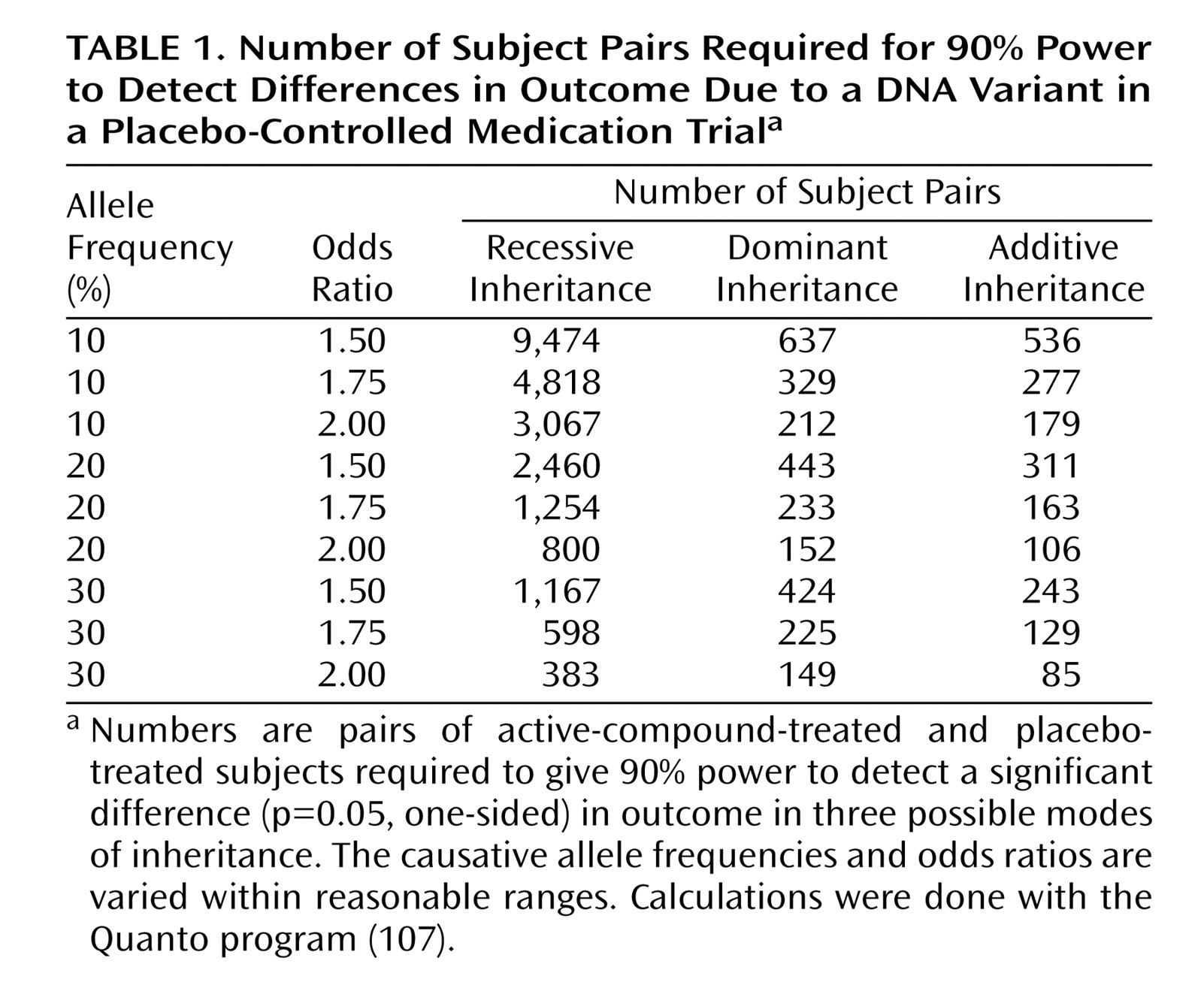
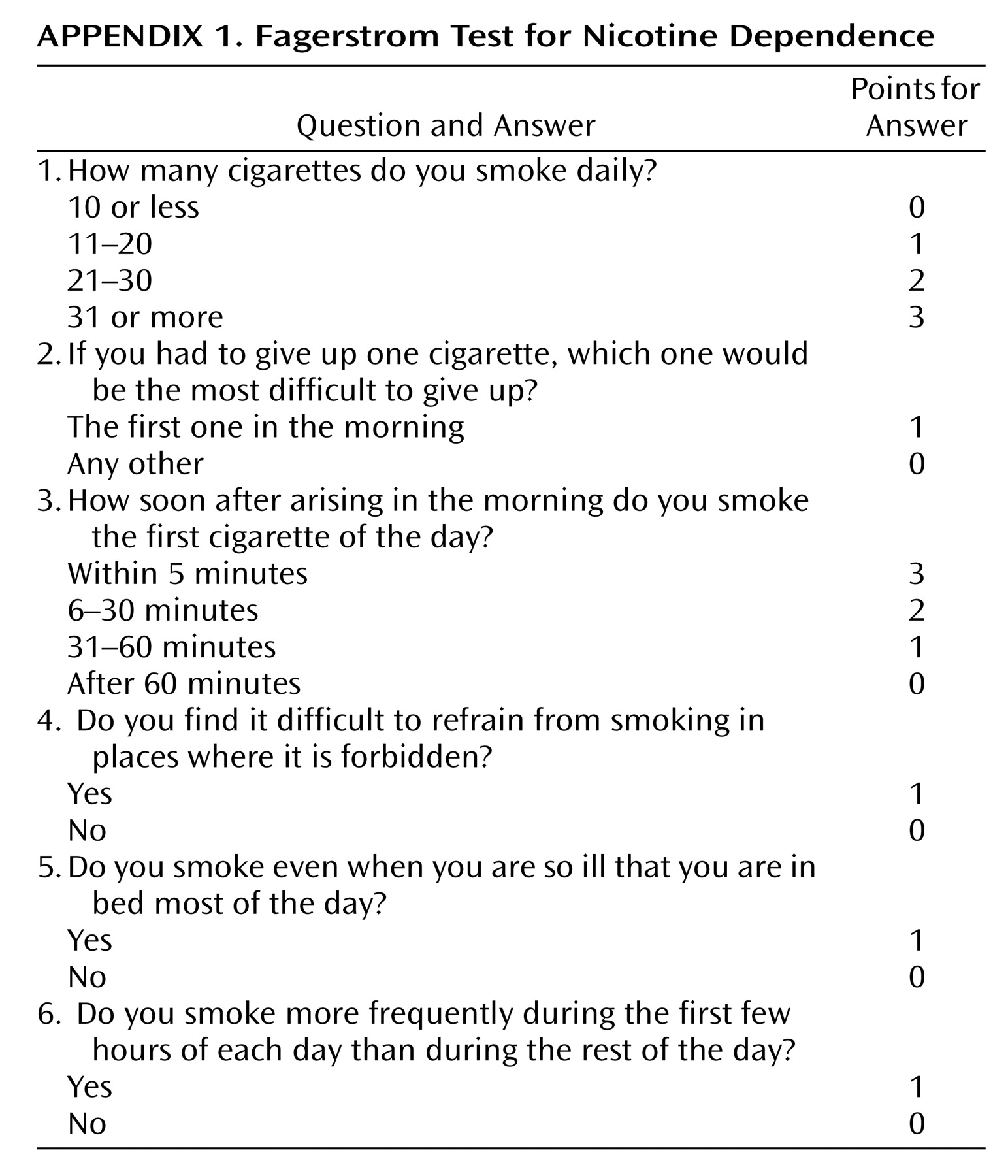
Once smoking becomes established (daily smoking for 1 month), approximately 20% of smokers develop nicotine dependence, based on responses to the Fagerstrom Test for Nicotine Dependence, which consists of six questions
(13) (
Appendix 1). Although the maximal score is 10, a score >4 indicates probable nicotine dependence.
Most nicotine-dependent persons report annual attempts to quit smoking, but less than 15% are successful over the long term
(5). To reduce tobacco-related morbidity and mortality, new approaches to nicotine dependence treatment are needed. Some of these new approaches may be developed on the basis of findings from the study of animal models of nicotine dependence.
Animal Models of Nicotine-Related Phenotypes
The rewarding effects of nicotine are mediated, in part, by release of dopamine in the nucleus accumbens from ventral tegmental area neurons
(14–
16). Increasing synaptic dopamine in the nucleus accumbens is a mechanism of reward for many abused drugs
(17). The mechanism through which nicotine mediates the increase in nucleus accumbens synaptic dopamine may involve the μ opioid receptor and its endogenous ligand, β-endorphin
(18,
19).
Several animal models have been developed for the rewarding valence of nicotine. Rodents can be trained to self-administer nicotine in an operant chamber, where a lever must be pressed to receive an intravenous bolus of nicotine. Drugs that reduce operant self-administration may show promise as smoking cessation medicines in clinical trials. One promising compound is baclofen, a γ-aminobutyric acid type B receptor antagonist, which has been shown to decrease intravenous self-administration of nicotine in rats
(20,
21). A second compound deserving additional study is a specific inhibitor of norepinephrine reuptake, reboxetine, which also reduced intravenous nicotine self-administration in rats
(22).
Another behavioral model of the rewarding valence of nicotine in rodents is conditioned place preference. This paradigm uses an apparatus consisting of a box divided into two distinct compartments, with a communicating door. The two compartments are distinguished by floors and walls of different appearance and/or texture (e.g., one compartment might have a wire mesh floor and vertical stripes on walls, while the other compartment has a solid plastic floor and horizontal dashes). Immediately after drug administration, which is usually by injection, the rodent is placed into one compartment, and the communicating door is locked. Exposure to the drug is paired consistently with the same compartment, with the communicating door locked, and placebo is paired with the other compartment. After several pairings of the drug with one compartment and placebo with the other over several days, the rodent (without any drug for the past day) is placed in the box with the communicating door open. If the animal spends more time on the drug-paired side, this result is taken as evidence that the animal experienced the drug as rewarding. Similarly, avoidance of the drug-paired side is evidence that the drug was aversive.
Drugs that block nicotine place preference in rodents may be useful compounds for smoking cessation trials in humans. This conceptual leap is often assumed to be valid because cigarette smoking is valued in part for its rewarding effects (e.g., mild euphoria). If a medication blocks the rewarding effects, it may reduce cigarette consumption.
However, drugs that universally block experience of reward for any event would not be acceptable compounds for clinical trials in smoking cessation, for obvious reasons. For example, a partial agonist at the strychnine-insensitive glycine receptor site blocks nicotine, morphine, cocaine, and amphetamine place preference in mice, but it does not interfere with place preference for sucrose pellets
(23). The absence of an effect on the natural reward of food suggests that the drug does not block all hedonic mechanisms in the brain.
Nicotine withdrawal may be studied by chronic nicotine administration in rodents, followed by a nicotinic antagonist injection
(24). Compounds that attenuate the severity of nicotine withdrawal symptoms in rodents may have promise in treatment of nicotine dependence because smoking may be maintained in part through avoidance of withdrawal.
Implicit in these studies is the assumption that drugs that reduce the rewarding valence of nicotine, or reduce nicotine withdrawal symptoms, may be useful clinically in treating nicotine dependence. Although this assumption may not be correct, it is supported by the use of nicotine replacement therapy as a means to manage nicotine withdrawal symptoms.
Studies of transgenic mice have provided some insights into the rewarding mechanisms of nicotine, and these findings have suggested pharmacological approaches to the treatment of nicotine dependence. Nicotine has been found to induce release of β-endorphin
(18,
19) and met-enkephalin
(25) from neurons. Mice lacking the μ opioid receptor gene, for which β-endorphin and met-enkephalin are naturally occurring ligands, do not show nicotine place preference
(26). Thus, some aspect of the rewarding valence of nicotine requires μ opioid receptors and (presumably) an endogenous ligand, either β-endorphin or met-enkephalin or both. These studies suggest a role for opioid antagonists in smoking cessation.
Mice lacking the β
2 subunit of nicotinic receptors do not experience nicotine as rewarding
(27). These data are consistent with the finding that the β
2 subunit is essential for nicotine to elicit dopamine release in the nucleus accumbens
(28). This assumption leads to the hypothesis that nicotine’s rewarding actions are mediated through binding to nicotinic receptors containing the β
2 subunit in the nucleus accumbens, yielding increased release of dopamine, with an essential role for a functioning μ opioid receptor system.
Mice lacking cannabinoid type 1 (CB1) receptors do not show nicotine place preference
(24). This study and other studies have led to the development of a promising compound for smoking cessation, the CB1 receptor antagonist rimonabant
(29).
Promising compounds for smoking cessation may also be identified by screening for blockade of nicotine-induced increases in synaptic dopamine in the nucleus accumbens, an action of nicotine that is essential to its rewarding valence
(15,
16). A recent example of this strategy is found in the observation that cannabinoid receptor antagonists can block the nicotine-induced release of presynaptic dopamine in the shell of the nucleus accumbens
(30).
In multiple instances, animal model studies have yielded promising compounds for clinical trials in nicotine dependence. These clinical trials will most likely lead to new pharmacotherapies approved by the United States Food and Drug Administration (FDA) for nicotine dependence in the near future. At present, however, only two pharmacotherapies for nicotine dependence have FDA approval: nicotine replacement and bupropion.
Pharmacogenetic Approaches to Nicotine Dependence Treatment
Despite progress made in the pharmacological treatment of nicotine dependence, the efficacy of available treatments is limited. Although current guidelines recommend transdermal nicotine as a first-line treatment for nicotine dependence
(31), the vast majority of smokers receiving transdermal nicotine relapse to their former smoking practices
(35,
36). Bupropion has been shown to produce higher quit rates than nicotine replacement therapy
(53), yet it is effective for only a minority of smokers.
Several studies have attempted to identify pretreatment variables that can be used to individualize pharmacotherapies for nicotine dependence, but with limited success. Measures of nicotine dependence, such as smoking rate, level of dependence, and cotinine level, have predicted outcome in some nicotine replacement therapy studies
(40,
74) but not in others
(75). Smokers with low to moderate nicotine dependence levels, nonobese smokers, and Caucasian smokers may benefit more from transdermal nicotine, and smokers who are highly dependent, obese, or members of minority groups may benefit more from nasal spray
(76). Bupropion may be a more effective treatment for women
(55) and smokers with higher levels of nicotine dependence
(58). These data do not provide an empirical basis on which to tailor the choice of treatment to individual smokers, but pharmacogenetic research may identify smokers for whom bupropion and nicotine replacement therapy will have the strongest benefit. Research on the role that inherited variation plays in the response to pharmacotherapy for nicotine dependence may yield individualized treatments based on genotype and may thereby improve efficacy.
A pharmacogenetic approach to nicotine dependence treatment may yield knowledge of DNA variants that influence treatment outcome, under the assumption that inherited differences in drug metabolism and drug targets influence toxicity and efficacy
(77–
79).
Pharmacogenetic Investigations of Nicotine Replacement Therapy
In a pharmacogenetic study conducted in the United Kingdom, 755 of 1,500 smokers participating in a placebo-controlled trial of transdermal nicotine also provided blood samples for DNA analysis
(80,
81). The DNA analysis focused on genetic variation in the dopamine pathway, on the basis of previous evidence that nicotine’s rewarding effects are mediated, in part, by dopaminergic mechanisms
(82). Transdermal nicotine was significantly more effective than placebo for carriers of the
A1 allele of the D
2 dopamine receptor gene (
DRD2) but not for
A2 homozygotes
(80). The difference in the odds ratios for the treatment effect between the genotype groups was significant after the first week of treatment but not at the end of treatment. This study also examined a polymorphism in the dopamine β-hydroxylase gene (
DBH). Transdermal nicotine was effective (odds ratio of 3.6 for transdermal nicotine versus placebo) in producing abstinence among smokers with both the
DRD2*A1 allele and the
DBH*A allele and was less effective for smokers with other genotypes. This genetic association with treatment response was significant at both 1 week and 12 weeks of treatment, suggesting that the efficacy of transdermal nicotine may be modulated by
DRD2 and
DBH. A follow-up study supported the association of the
DRD2*A1 variant with abstinence at 6 and 12 months posttreatment; however, the effect was observed only among women. Results for
DBH were not reported
(81).
In an open-label pharmacogenetic trial of transdermal nicotine versus nicotine nasal spray, the role of the μ opioid receptor gene (
OPRM1) gene was examined
(83). The μ opioid receptor is the primary site of action for the rewarding effects of the endogenous opioid peptide, β-endorphin, which is released afer acute and short-term nicotine administration
(18,
19). Exon 1 of the human
OPRM1 gene includes a common Asn40Asp (A118G) mis-sense single nucleotide polymorphism. The Asp40 variant increases the binding affinity of β-endorphin for this receptor threefold, relative to the wild-type Asn40
OPRM1 (84). The Asp40 variant is found in about 25%–30% of individuals of European ancestry
(85,
86) and is therefore sufficiently common to explain clinically significant differences in response to different forms of nicotine replacement therapy.
Among 320 smokers of European ancestry, persons carrying the
OPRM1 Asp40 variant (Asn/Asp or Asp/Asp, N=82) were significantly more likely than those homozygous for the Asn40 variant (Asn/Asn, N=238) to be abstinent at the end of the treatment
(83). The differential treatment response was evident among smokers who received transdermal nicotine (quit rates of 52% versus 33% for the Asp40 and Asn40 groups, respectively; odds ratio=2.4) but was not significant among smokers who received nicotine nasal spray. Among smokers who received transdermal nicotine, the advantage for the Asp40 group was significantly greater during the 21-mg dose phase. Smokers with the Asp40 variant also reported significantly less severe withdrawal symptoms and mood disturbance during the first 2 weeks of abstinence and gained significantly less weight at the end of treatment, compared to those with the Asn40 genotype. Although these results must be validated in future research, the findings suggest a hypothesis that smokers with the
OPRM1 Asp40 variant may achieve significant benefit from transdermal nicotine and may be candidates for extended high-dose patch treatment, or even maintenance therapy, to reduce risk of relapse.
Consistent with this pharmacogenetic hypothesis, a longitudinal analysis in the transdermal nicotine group revealed that the
OPRM1 genotype effect in the Asp40 group was greatest during 21-mg patch treatment, declined as treatment was tapered, and disappeared after treatment was discontinued. In the Asn40 homozygotes, dose tapering did not appear to alter abstinence rates, which declined steadily from the quit date. Event history analysis of lapse and recovery events showed that transdermal nicotine-treated smokers with the Asp40 variant were significantly more likely to
recover from lapses than were Asn40
homozygotes during the 21-mg dose phase
(83). There was no genotype effect on recovery from lapses during the 14-mg or 7-mg phase or after treatment was discontinued. Thus, nicotine-dependent persons with the Asp40 allele may benefit from extended higher-dose transdermal nicotine therapy.
Consistent with the treatment outcome data from this trial
(83), smokers with the
OPRM1 Asp40 variant reported significantly less severe withdrawal symptoms and mood disturbance during the first 2 weeks of abstinence, compared to the Asn40
homozygotes. An increase in negative affect during this period strongly predicted relapse. Smokers with the Asp40 variant also had significantly less weight gain at the end of treatment than did the Asn40 homozygotes.
OPRM1 genotype effects on these intermediate outcomes may be mediated by greater β-endorphin occupancy at the μ receptor. Although these results must be validated in future research, the findings suggest a hypothesis that smokers with the
OPRM1 Asp40 variant may be candidates for extended high-dose patch treatment, or even maintenance therapy, as an alternative to smoking.
Pharmacogenetic Investigations of Bupropion
A placebo-controlled smoking cessation clinical trial of bupropion
(87) was the source of pharmacogenetic analyses focused on the cytochrome P450 2B6 gene (
CYP2B6), which has been implicated in bupropion kinetics
(88) and in brain nicotine metabolism
(89). In this trial, 426 smokers of European ancestry provided blood samples and received bupropion (300 mg/day for 10 weeks) or placebo, plus counseling. Smokers with decreased activity alleles of
CYP2B6 (slower metabolizers) reported greater increases in cravings for cigarettes following the target quit date and had significantly higher relapse rates. These effects were modified by a significant gender-by-genotype-by-treatment interaction, suggesting that bupropion attenuated the effects of genotype among female smokers. The findings of a significant association of
CYP2B6 genotype with smoking cessation in the placebo group and absence of a genotype association with bupropion side effects suggest that the genotype effect on treatment outcome is not attributable to bupropion pharmacokinetics. Rather, the greater relapse liability in the genetically slower metabolizers may be attributable to slower rates of inactivation of nicotine (by conversion to cotinine) in the central nervous system. Additional trials are warranted to confirm these results, as are studies to explore the neurobiological mechanisms of the observed genetic effect.
A second report from this clinical trial
(87) examined genetic variation in the dopamine pathway, based on the premise that bupropion’s effects are attributable, in part, to inhibition of dopamine reuptake
(46). The genetic analysis focused on common polymorphisms in the dopamine transporter (
SLC6A3) gene and the
DRD2 gene, both of which had been associated in previous studies with smoking behavior
(90–
93). Although the analysis did not support the hypothesis of genetic modulation of response to bupropion, the results revealed a significant gene-gene interaction effect on liability to relapse, mirroring results from a previous study of smoking status
(90). Specifically, smokers with
DRD2*A2 and
SLC6A3*9 alleles had significantly higher abstinence rates at the end of treatment (53% versus 39%) and a longer latency to relapse at the end of treatment (28 versus 21 days) and at 6-month follow-up (83 versus 65 days). By contrast, among smokers with
DRD2*A1 genotypes, the effect of
SLC6A3 on abstinence rates and time to relapse was not significant.
On the basis of existing biological and epidemiological data on
DRD2 and
SLC6A3, a biobehavioral mechanism for the observed findings can be postulated. Data suggest that individuals with
DRD2*A1 genotypes exhibit lower dopamine D
2 receptor density and, therefore, may have lower levels of neuronal dopamine-dependent activity, compared to individuals with
DRD2*A2 genotypes
(94–
96). This interpretation is consistent with epidemiological evidence for association of
DRD2*A1 with cognitive function
(97,
98), as well as with a variety of addictive behaviors
(99). With regard to
SLC6A3, the 9-repeat genotype has been associated with lower levels of dopamine transporter protein expression
(100,
101). This lower level of expression may yield less neuronal dopamine reuptake and higher levels of synaptic dopamine. Thus, individuals with this 9-repeat allele may derive less reinforcement from nicotine-induced dopamine release, by virtue of a chronically high level of synaptic dopamine at baseline. Thus, one could speculate that in the presence of normal receptor function (i.e., in individuals with
DRD2*A2 genotypes), lower dopamine transporter levels and higher levels of synaptic dopamine (i.e., in individuals with
SLC6A3*9 genotypes) would minimize the phasic effects of nicotine on dopamine release, thereby reducing positive reinforcement from smoking. This effect, in turn, would make it easier for smokers with the
DRD2*A2/
SLC6A3*9 haplotype to maintain smoking abstinence. By contrast, individuals with lower synaptic dopamine levels (i.e., those with
SLC6A3*10 genotypes) and normal receptor density (i.e., those with
DRD2*A2 genotypes) might have the greatest need for and reinforcement from nicotine.
An association of the
DRD2 Taq1 polymorphism with bupropion’s effects on subjective withdrawal symptoms has also been reported in a small investigation
(102). The
A1 allele of
DRD2 has also been linked with smoking cessation and abstinence-induced negative mood symptoms following treatment with venlafaxine, a serotonin and norepinephrine reuptake inhibitor
(103).
Weight gain occurs in a majority of daily smokers after quitting
(104,
105), and weight gain predicts relapse to smoking
(105). To investigate possible mechanisms, we examined genetic variations in the dopamine pathway as moderators of the effect of bupropion on abstinence-induced changes in the rewarding value of food
(106). This analysis was based on the evidence described earlier for the beneficial effects of bupropion on weight gain after smoking cessation
(51). Seventy-one smokers of European ancestry participated in this experiment; all of them were genotyped for the
DRD2 Taq1 polymorphism and randomly assigned to treatment with bupropion (300 mg/day) or placebo. They participated in two behavioral laboratory sessions during which the rewarding value of food was assessed with a behavioral economics measure: session 1 occurred before medication and before cessation of smoking; session 2 occurred after 3 weeks of bupropion treatment and 1 week of sustained abstinence. Carriers of the
DRD2*A1 allele exhibited significant increases in the rewarding value of food after abstinence from smoking, and these effects were attenuated by bupropion treatment (a significant medication-by-genotype interaction). Further, higher levels of food reward at session 2 (postquit) predicted a significant increase in weight by 6-month follow-up in the placebo group but not the bupropion-treated group. These results provide new evidence that the increase in body weight that occurs after smoking cessation is related to increases in food reward and that food reward is partly determined by genetic factors. Bupropion’s efficacy in attenuating abstinence-induced weight gain may be attributable, in part, to its effect in decreasing food reward.
Implications of Pharmacogenetic Research in Nicotine Dependence for Psychiatry Practice
Individuals with psychiatric illness carry a disproportionate burden from cigarette smoking and tobacco-related mortality. Data from a nationally representative survey indicated that individuals with a current psychiatric illness are not only more likely to be regular smokers but also less likely to quit smoking
(108). Ninety-two percent of persons with a diagnosis of schizophrenia have been estimated to have a lifetime history of smoking and 83% to be current smokers, compared to about one-quarter of the general population
(109). Rates of current smoking are also elevated among persons with bipolar disorder (69%), major depression (37%), and generalized anxiety disorder (46%)
(108). Further, there is evidence for a common genetic etiology for smoking and psychiatric illness and support for the role of the α
7 neuronal nicotinic receptor in both schizophrenia and bipolar disorder
(110).
Although smoking cessation treatments have not been extensively studied in persons with psychiatric illness, some pharmacotherapies have shown promise. For example, in a randomized clinical trial of a smoking cessation intervention for persons with schizophrenia, bupropion was found to be safe and significantly more effective than placebo
(111). Evidence for the safety and efficacy of transdermal nicotine, especially in conjunction with atypical antipsychotic agents, has been reported
(112).
Pharmacogenetics research in smoking has the potential to advance the science and practice of smoking cessation treatment in the general population and among persons with psychiatric illness. In addition to providing insights into targets for novel pharmacotherapies, information about smokers’ genotypes may allow practitioners to select the optimal type, dosage, and duration of treatment for individual patients. Although this research is still in its infancy, recent data suggested that health care providers are very favorably disposed toward providing genetically tailored treatment in practice; for example, among 1,120 physicians in the American Medical Association, the average reported likelihood of adoption of genetic testing to tailor smoking treatment (on a 0%–100% scale) was 73.5%
(113). However, several barriers to clinical translation of pharmacogenetics research in smoking must be addressed, including a lack of preparedness of health care providers to counsel patients about genetic results and concerns regarding the potential for stigmatization and discrimination based on genetic findings
(113,
114). Such findings highlight the importance of provider education in genetics, as well as the need to address broader health care policy issues.
Summary
Despite 40 years of government warnings regarding the negative health consequences of smoking, roughly 23% of adults in the United States are daily tobacco smokers. Nicotine produces a dependence syndrome that renders abstinence a difficult goal. Social pressures against smoking have increased, producing fewer opportunities to smoke, yet smoking represents the single largest preventable source of morbidity and mortality in the United States. Thus, the need for new medications is great.
New medications for nicotine dependence may be developed through animal models of the reinforcing value of nicotine as well as through pharmacological studies of nicotine withdrawal. Studies of transgenic rodents have been valuable in identifying specific genes whose proteins can be targeted for pharmacotherapy.
The initial pharmacogenetic studies of FDA-approved pharmacotherapies, including nicotine replacement and bupropion, have appeared in the literature. These studies have identified (in a preliminary manner) candidate alleles at the D2 dopamine receptor gene and μ opioid receptor gene that may predict therapeutic response. Given the complexity of the nicotine dependence phenotype, it seems that no one medication will show efficacy and safety for a majority of nicotine-dependent individuals. Thus, it will be essential to use genetics and other tools to predict therapeutic response in subgroups of nicotine-dependent persons.
This research is of particular importance to psychiatrists, as a large fraction of individuals with behavioral disorders are daily smokers, including the majority of persons with schizophrenia and affective disorders
(2). Psychiatrists must consider smoking cessation therapy a high priority in their clinical practices, and newer, more efficacious medications will facilitate this change.