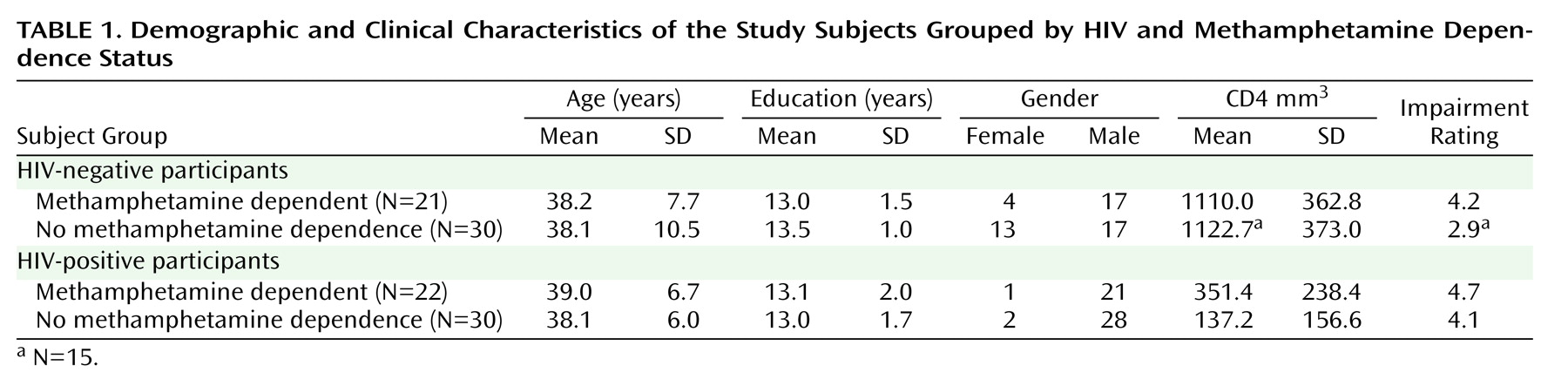
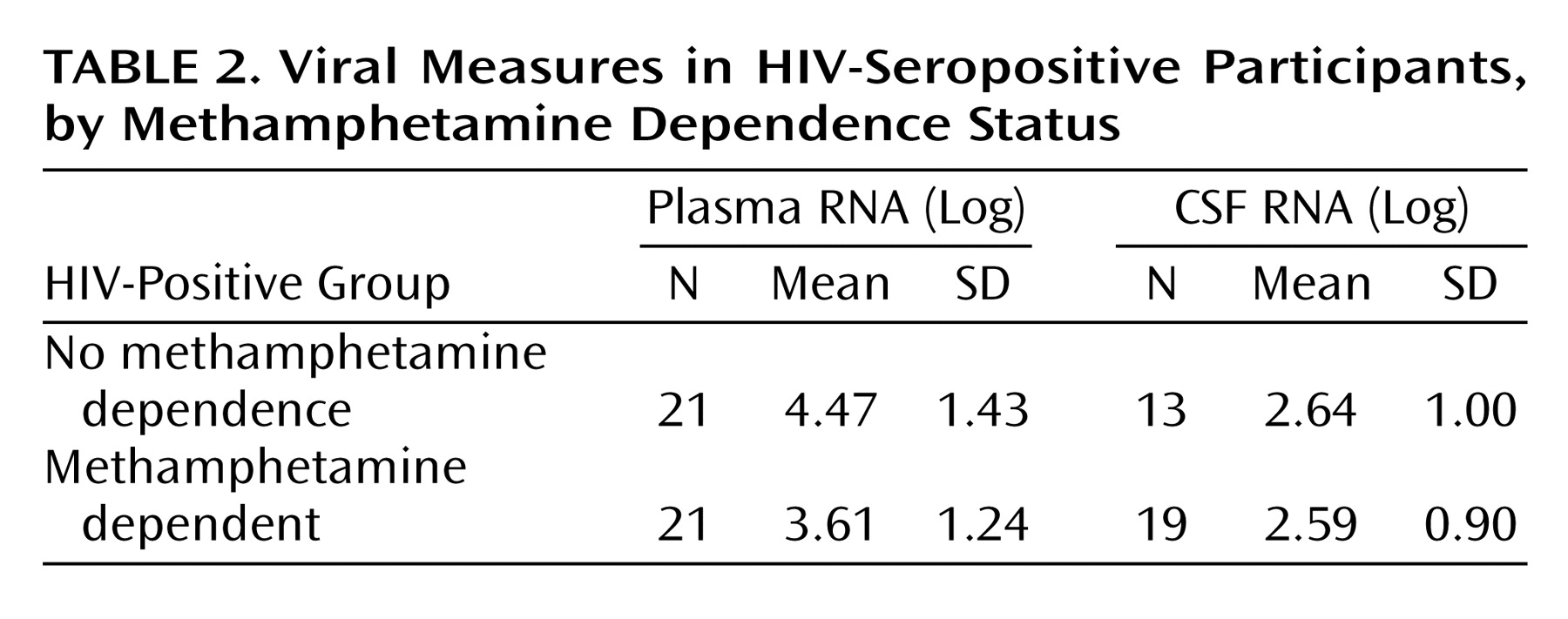
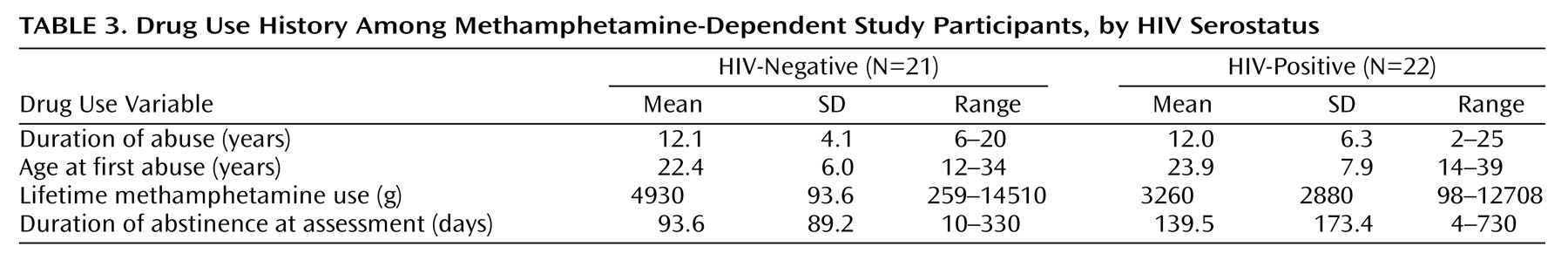
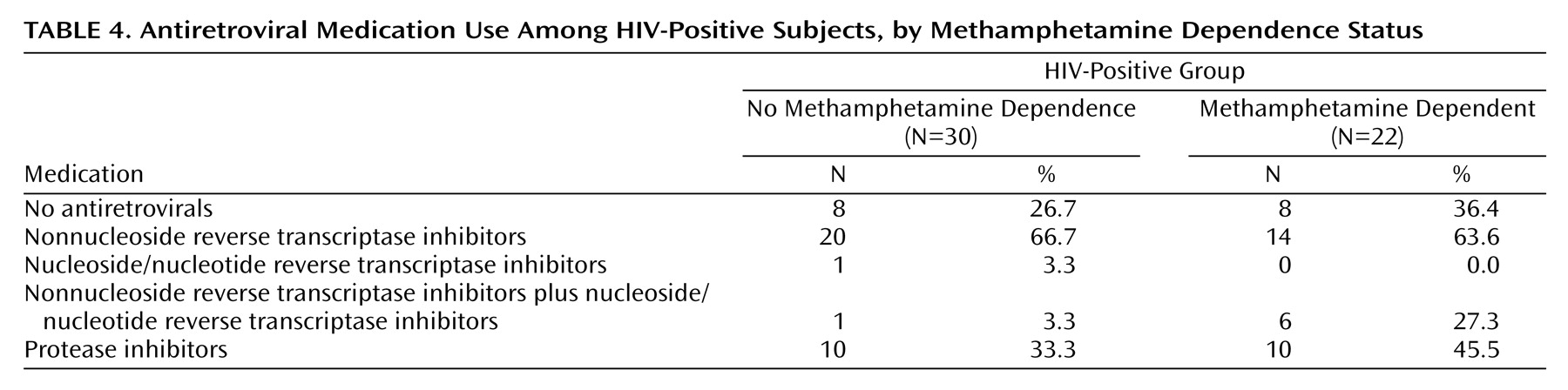
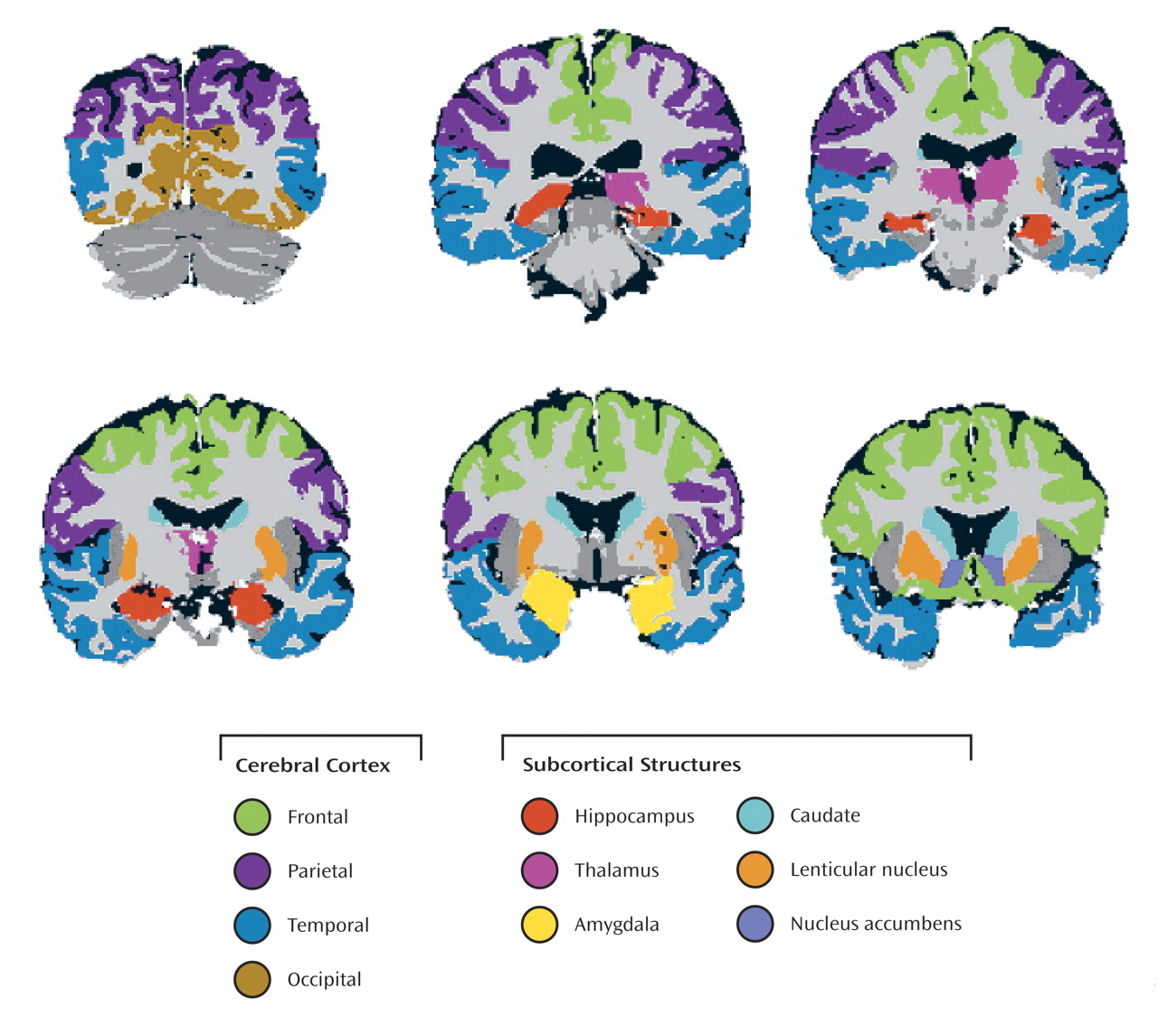
Methamphetamine Effects
In this study, comparisons of groups of individuals with and without methamphetamine dependence revealed unexpected basal ganglia and parietal cortex volume increases. Striatal structures, most notably the nucleus accumbens, have been implicated in previous studies of the CNS effects of stimulants. A number of studies have focused on mechanisms by which behavioral and neural sensitization occurs as a result of stimulant exposure (see Steketee
[20] for a review), since such sensitization has putative links to the development of dependence and craving. These studies have demonstrated that low doses of stimulants such as cocaine and amphetamine result in increased dopamine release in the nucleus accumbens that is further enhanced as a result of repeated, intermittent exposure
(21–
23). Animal studies
(24,
25) have shown that acute doses of methamphetamine that mimic binge doses in human abusers result in neurotoxic effects on striatal dopamine terminals; i.e., reduced dopamine and metabolite levels and reduced dopamine transporter binding. However, when these doses are preceded by escalating lower doses, as plausibly occurs in human users, the effects on dopamine terminals, while still present, are substantially mitigated and show greater recovery.
In other animal studies, Robinson and Kolb
(26,
27) have shown that exposure to low-dose amphetamine in rats is followed by long-lasting increases in the length of dendrites, density of dendritic spines, and number of branched spines on output cells of the nucleus accumbens. Similar effects were observed in the prefrontal cortex. In previous studies
(28,
29) the effects of methamphetamine exposure on plasticity-related genes in the rat brain were examined. Such exposure resulted in increases by 20%–40% in the expression of synaptophysin mRNA in the nucleus accumbens and in prefrontal and temporal cortices within 1 day of administration. Increases in stathmin mRNA were also observed in the prefrontal cortex. Thus, increases in expression of plasticity-related genes may lead to previously reported neurite extension in the striatum, and these changes may contribute to the volume increases observed in the present study.
Another relevant study
(30) examined the effects of MPTP-induced dopaminergic lesions on tyrosine hydroxylase fibers in monkeys. It is interesting that some fibers became thicker and increased their branching, and mRNA for growth-associated protein-43 was upregulated in midbrain dopamine cells. The authors concluded that sprouting in the striatum was a compensatory response to partial dopaminergic depletion. Thus, multiple lines of evidence suggest that some progressive compensatory changes, perhaps associated with the depletion of dopamine transporters or damage to dopamine terminals, could contribute to the volume increases observed in methamphetamine-dependent individuals.
In addition to these animal studies of sprouting in the striatum, several studies have demonstrated pronounced effects on microglial activation in methamphetamine-exposed rodents
(31,
32), both in the striatum and, specifically, in the somatosensory cortex; these studies have suggested a role for microglial activation in methamphetamine neurotoxicity. Microglial activation has also been linked to astrocytosis
(33), and both astrocytosis and depletion of glutamate-staining neurons have been observed in the somatosensory cortex of methamphetamine-exposed animals
(34). Finally, Eisch et al.
(35) examined [
3H]mazindol ([
3H]MAZ) binding to striatal dopamine transporters and [
3H]GLU binding to
N-methyl-
d-aspartate (NMDA) receptors in the striatum and cortex 1 week and 1 month after a neurotoxic methamphetamine regimen. Dopamine transporters and NMDA receptors were reduced in the striatum after 1 week, and decreases in dopamine transporters but not NMDA receptors persisted at 1 month. Of interest is that increases in NMDA receptors in the somatosensory cortex (but not the frontal cortex) were present at both time points, possibly increasing the vulnerability of this area to excitotoxicity.
In summary, methamphetamine exposure in rodents has been shown to 1) reduce striatal and frontocortical dopamine transporters, 2) lead to neuroplastic changes in the striatum as well as microglial and astrocytic activation in the striatum and parietal cortex, and 3) affect parietal lobe NMDA receptors and glutamatergic neurons. Thus, it appears that microgliosis, astrocytosis, and neuroplastic changes associated with methamphetamine exposure may underlie the striatal volume increases we observed. Methamphetamine-related microgliosis, astrocytosis, and glutamatergic excitotoxicity, observed specifically in parietal regions in animal studies, may occur in the methamphetamine abusers studied here and may contribute to their parietal lobe volume increases.
Previous PET studies in stimulant-dependent individuals have also specifically implicated striatal and parietal lobe structures, where depletion of striatal dopamine transporters and reduced striatal metabolism have been observed, as well as some recovery of striatal dopamine transporters with abstinence
(36–
40). Although in the PET studies striatal regions showed significantly reduced
relative cerebral metabolism on PET, global cerebral metabolism was shown to be increased in recovering methamphetamine abusers, and of interest is that this increase appeared to be disproportionately due to increased metabolism in the parietal cortex
(37).
MR spectroscopy studies in human abusers also suggest that methamphetamine-related neurotoxicity may result in neuroinflammatory responses and astrogliosis
(41). Such factors could contribute to apparent volume increases in affected structures on MRI, either through true volume increases or by affecting the MR signal of the local white or gray matter such that the volumes appear to be larger.
Last, it is also possible that the structural anomalies observed here in methamphetamine-dependent participants do not result from stimulant exposure but instead are markers of the vulnerability of these individuals for the development of substance use disorders. Of particular concern in this regard is the high comorbidity of substance dependence and developmental disorders such as attention deficit hyperactivity disorder and antisocial personality disorder. Although such comorbidity was present in the current sample, no evidence emerged for a link between either and the volume increases noted. However, developmental factors that preceded the stimulant exposure in these participants cannot be ruled out as an explanation. Longitudinal studies of these subjects may yield additional information, if the changes can be linked to use patterns over time.
While increased structural volumes have not been reported previously in methamphetamine abusers, it should be noted that similar findings have been reported in cocaine abusers. Jacobsen et al.
(42) measured caudate and putamen volumes in cocaine abusers and reported increases of 3.4% and 9.2%, respectively, in the abusers relative to comparison subjects. These authors also speculated that the volume increases they observed might be related to reductions of striatal dopamine transporters.
It is interesting that other studies focusing on the cerebral cortex have provided evidence of volume loss in cocaine abusers, specifically in prefrontal and temporal lobe regions
(16,
43). Thompson et al.
(17) found limbic volume decreases in active methamphetamine users. Reductions in cortical and limbic structures were not observed in this sample of methamphetamine-dependent individuals. It is possible that the effects of methamphetamine dependence on the cerebral cortex may differ from those of other stimulants, and effects in active users may differ from those in recovering abusers. Alternatively, neurodegenerative changes in the present methamphetamine-positive group may have been obscured by mechanisms related to the volume increases we observed in the parietal cortex.
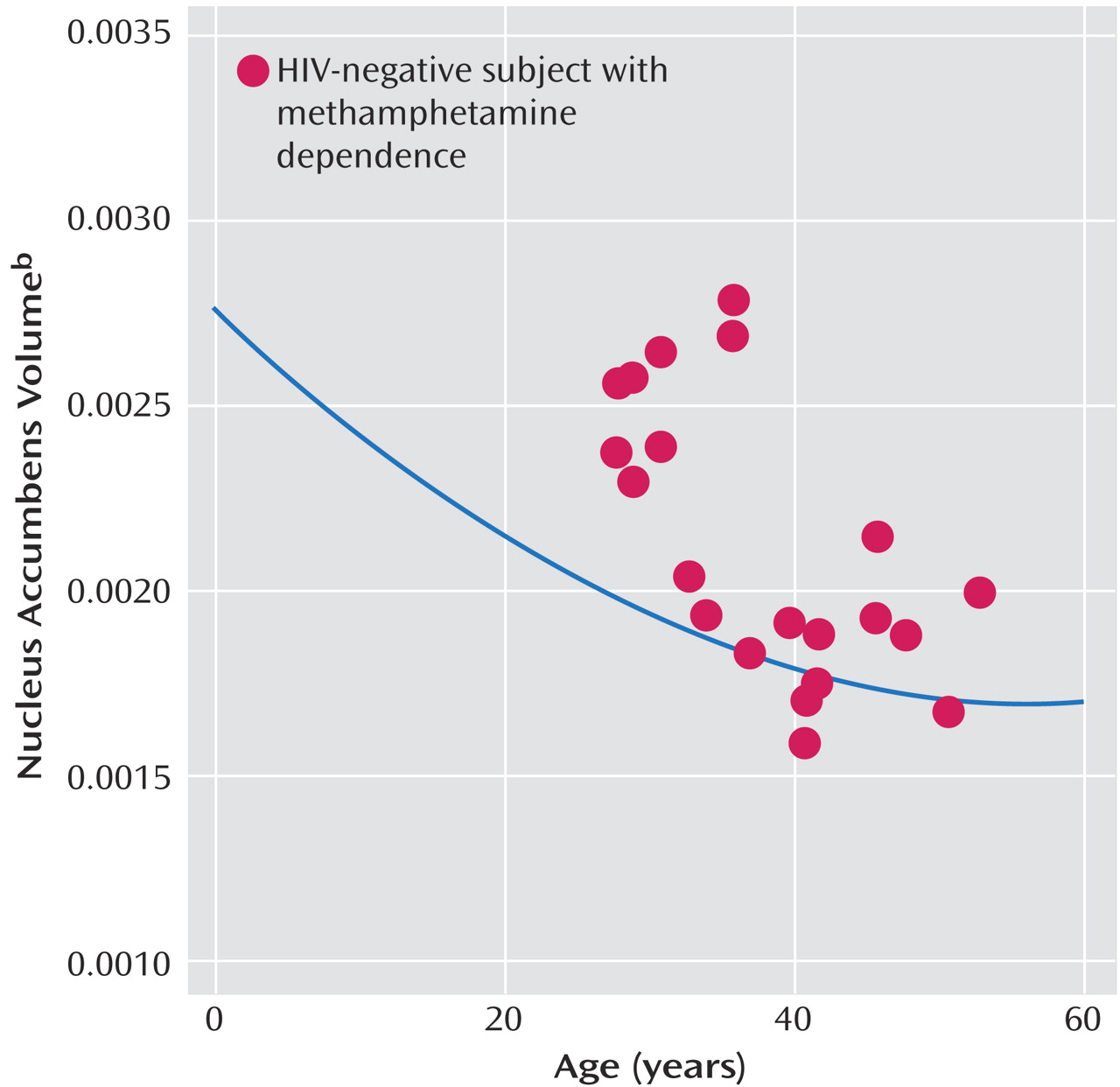
There was evidence in the present study that the volume increases in the nucleus accumbens were more pronounced in younger methamphetamine-dependent participants. Such age-related variability in the effect of methamphetamine dependence warrants further examination. It is possible that methamphetamine effects on brain volumes are biphasic, with more prominent “trophic” effects during the early course of dependence, and more prominent neurodegenerative effects with longer periods of dependence. However, estimates of duration of dependence did not correlate with brain volumes in our study.
It is also possible that the prominence of volume increases in younger subjects occurs because the younger brain responds differently to methamphetamine exposure. In the present study, estimated age at first exposure was strongly correlated with the current age of the subjects. Thus, it was difficult to assess whether the apparent age dependence of the changes was mediated by the subjects’ present age or by the age at which regular exposure began.
Previous studies of late brain maturation during adolescence and early adulthood
(44–
46) have provided evidence for continuing myelination in white matter, and for continuing volume loss in cerebral gray matter structures—notably in the striatum. Methamphetamine exposure during adolescence may alter the course of ongoing brain maturation in these structures. In
Figure 3, the (proportionalized) nucleus accumbens volumes of the HIV-negative/methamphetamine-dependent subjects in the present study are plotted against age. The curve shows the function relating the normalized nucleus accumbens volumes (measured using the same methods applied in the present study) to age in normally developing youngsters and young adults found in our previous studies
(19,
45). As can be seen, significant volume reduction in the nucleus accumbens occurs in normally developing children. This figure illustrates that while the volumes observed in the younger methamphetamine-dependent subjects are increased relative to those of their nonabusing age-peers, they are more similar to volumes measured in 10–15-year-old normal youngsters.
Combined Effects of HIV and Methamphetamine
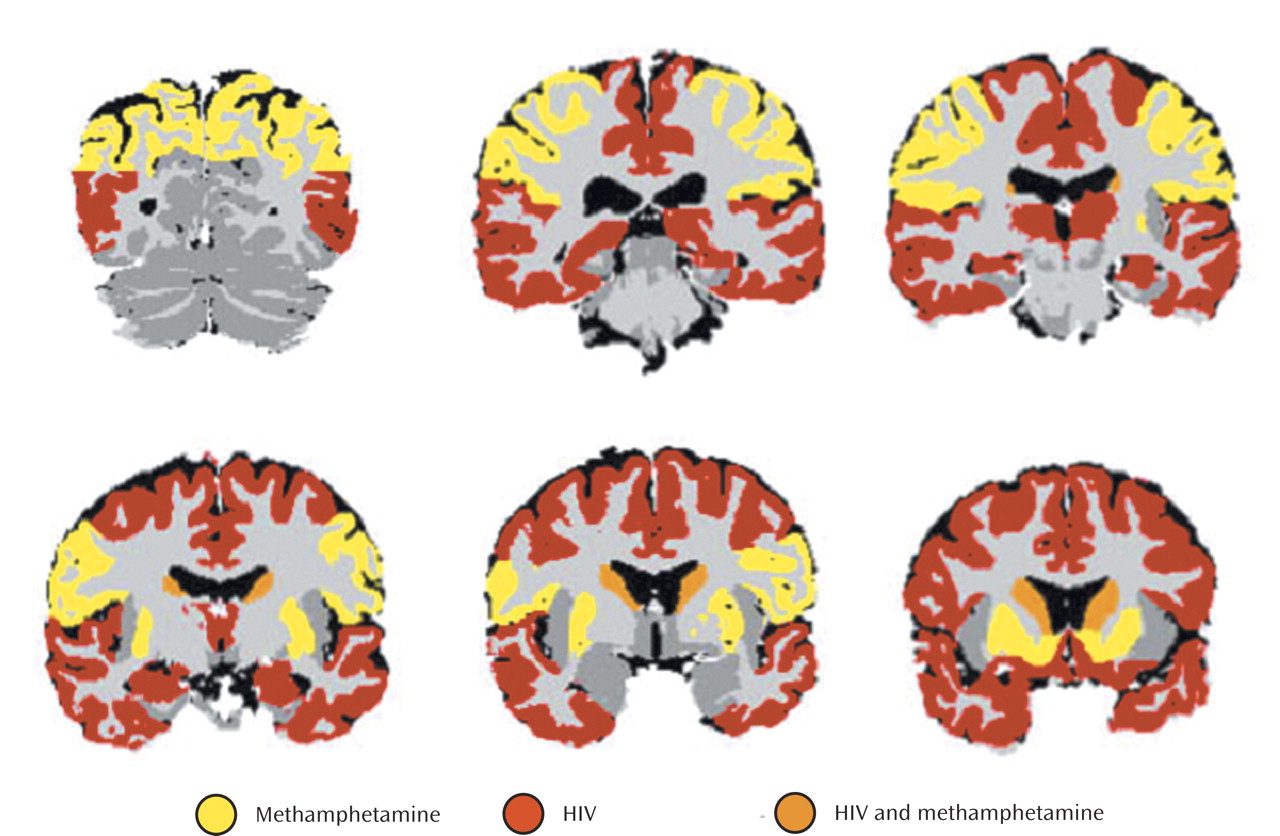
These results suggest overlapping, and opposing, effects of methamphetamine dependence and HIV infection on brain volumes. Apparently, in some brain structures, the effects of one or the other factor is dominant and this results in our report of different regional patterns associated with HIV effects and methamphetamine effects. In at least one site within the brain, the caudate nucleus, the effects of both factors are sufficiently strong to be detectable. In this structure, the effects, when combined, are likely to yield apparently normal volumes. This complicates the interpretation of striatal volumes in dually affected individuals. Furthermore, whatever the mechanisms underlying the volume increases and decreases we observe, they are likely to be associated with highly complex functional interactions within both affected and unaffected structures.
We did not observe statistically significant HIV status-by-methamphetamine interaction effects. That is, we did not obtain evidence for a significantly different effect of methamphetamine in HIV-positive than in HIV-negative individuals, nor of a significantly different effect of HIV in individuals with and without methamphetamine dependence. Rather, we obtained evidence for an additive effect of the two factors in the dually affected group. It is not possible to determine from these data whether the effects of methamphetamine and HIV are mediated by similar or completely different mechanisms. It is even possible, given these data, that the effects of HIV and methamphetamine are competitive (i.e., the effects of methamphetamine reverse those of HIV), although this seems very unlikely, since evidence from this study and a previous study
(1) suggests that dually affected individuals have more severe neurocognitive impairment. The lack of significant HIV status-by-methamphetamine interaction effects on brain structure does not imply that functional interaction effects do not occur in association with those mechanisms that alter morphology. It is even possible that significant interaction effects on brain volume could emerge from analyses with larger samples.