Recent estimates indicate that the number of new cocaine users per year more than doubled from 1992 (0.5 million) to 2002 (1.1 million)
(1). From 1994 to 2001, visits to hospital emergency rooms that involved cocaine increased by 22%
(2). Despite more than three decades of research into the molecular, cellular, and behavioral effects of cocaine, no pharmacotherapy for cocaine abuse has demonstrated sufficient safety and effectiveness for widespread clinical use
(3,
4). As astutely pointed out by Leshner
(5), most people see drug abuse and addiction as a social problem, and as such, the problem is frequently handled only with social solutions, in particular through the criminal justice system. Recent reports indicate that the 40%–60% of people seeking treatment are ordered to do so by the courts or corrections system (see reference
6), suggesting that treatment does not occur until drug use has led to substantial adverse consequences to the individual. However, if drug addiction is viewed as a brain disease, then addiction should be amenable to treatments, just as depression, schizophrenia, and anxiety are treatable. As Leshner stated,
Use of Brain Imaging Techniques in Cocaine Abuse Research
Cocaine is an indirectly acting monoamine agonist, binding to the dopamine, serotonin, and norepinephrine transporters
(8,
9). The majority of research on cocaine has involved the dopamine system, which will be the focus of this review. Cocaine binds to the dopamine transporter and blocks uptake of dopamine from the synapse. The elevated levels of dopamine activate two superfamilies of dopamine receptors, D1- and D2-like. This review will focus on D2-like receptors, which have been intimately linked to drug abuse (for instance, see references
10–12). The positron emission tomography (PET) studies that we will describe involve radiotracers that do not differentiate among subtypes of the D2 superfamily (i.e., D
2, D
3, and D
4 receptor subtypes).
To study the brain, powerful imaging techniques have been developed that allow for the noninvasive exploration of brain function in humans and in animals. In this review, we will highlight studies utilizing PET. A clear strength of PET is the ability to examine the brain repeatedly in longitudinal studies. PET has been described as a functional measure of brain activity because it records the uptake and washout of a radioactive marker that competes with endogenous neurotransmitters
(13). For example, the radiotracers [
11C]raclopride and [
18F]fluoroclebopride bind to D2-like receptors with similar affinities. The primary dependent variable in PET imaging studies is the ratio of the distribution of radioligand in the region of interest compared to its distribution in a region devoid of receptors. This ratio, termed the distribution volume ratio, provides a unitless number representing the ratio of receptor density (B
max) to receptor affinity. In theory, changes in the distribution volume ratio noted in longitudinal studies reflect changes in B
max. However, changes in the distribution volume ratio may also reflect changes in extracellular dopamine levels. Increases in extracellular dopamine will decrease radioligand binding, whereas decreases in extracellular dopamine will increase radioligand binding (for example, see references
13–15). Thus, in addition to being affected by the actual number of receptors in the tissue, the binding of these radioligands is influenced by the amount of dopamine present in the synapse. As a result, when we describe PET data, we use interchangeably “binding” and “availability,” since both are being assessed.
In contrast to PET imaging studies, in vitro receptor autoradiography provides a measure of receptor density in a particular brain structure that is not influenced by the levels of a neurotransmitter. The limitation, however, is that the procedures are terminal and, consequently, only one time point can be studied. These are important considerations for longitudinal PET studies because the possibility that changes in PET measures are due to changes in neurotransmitter levels rather than the density of receptors cannot be ruled out unless additional studies are conducted. In this review, we attempt to address this issue by also describing in vitro studies using receptor autoradiography procedures in which circulating dopamine levels are not influencing receptor density assessments.
Animal Models of Drug Abuse: One of the Most Powerful Models of Human Disease
One strategy implemented by the National Institute on Drug Abuse is the development of novel animal models to better understand the neurochemical mechanisms mediating the high abuse potential of cocaine. A greater understanding of the neuropharmacology of cocaine will ultimately lead to improved treatment for cocaine dependence. Animal models of drug self-administration have proven to be valid predictors of human drug abuse
(3,
16–20). Animals will self-administer most of the drugs that humans abuse, including cocaine, alcohol, nicotine, and heroin, by the routes (intravenous, inhaled, and oral) used by humans
(17). More recently, these models have been extended to examine behavioral and physiological responsiveness to environmental variables as indicators of vulnerability to drug use
(21). In one of the earliest studies on individual differences and vulnerability to drug abuse, Piazza et al.
(22) initially characterized rats’ behavior in an open-field apparatus on the basis of how much locomotor activity was observed prior to any drug exposure. They found that rats characterized as “high responders” in an open field had higher basal corticosterone levels and were more likely to self-administer
d-amphetamine than rats characterized as “low responders.” Consistent with this characterization, noncontingent electric foot shock, which increased corticosterone levels in rats, facilitated acquisition of cocaine self-administration
(23). In other experiments, bilateral adrenalectomy or administration of metyrapone, which blocks corticosterone synthesis, completely abolished the acquisition of cocaine self-administration
(24). Thus, it appears that individual differences in vulnerability to drug abuse can be observed in animal models and that these differences may be due, in part, to stress-hormone responsiveness.
In addition, animal models of drug abuse have begun to incorporate more sophisticated designs, including the inclusion of alternative reinforcers and the study of cocaine choice, that may provide greater validity than models that simply examine the reinforcing effects of drugs (see references
25–27). For example, when monkeys are given a choice between food and cocaine, there is a dose-dependent relationship between drug dose and preference (see, for instance, references
26–28). That is, when low cocaine doses are the alternative to food, monkeys choose food; at higher cocaine doses, a monkey’s preference shifts to almost exclusively cocaine choices. Manipulations of environmental variables can increase or decrease cocaine choice. For example, increases in the magnitude of the nondrug alternative (i.e., food reinforcement) decrease cocaine choice. Similarly, increases in the response requirement for cocaine (analogous to increases in the cost of the drug) decrease cocaine choice
(26,
27,
29). The converse is also true: increases in the cost of the preferred nondrug alternative increase the frequency with which cocaine is chosen
(26,
27,
29).
These results emphasize that drug reinforcement is not simply mediated by actions in the brain but that the environment can alter drug reinforcement and, as we will show, brain function. A growing body of preclinical research supports the hypothesis that environmental stressors can enhance and environmental enrichment can attenuate the reinforcing effects of drugs. This hypothesis has clear implications for clinical outcomes. As we will discuss, these environmental stimuli affect brain function, including processes mediated by dopamine receptors. Compared to enrichment, more work has focused on the role of stress in drug abuse (see reference
30), with stressors including foot shock or more ethologically relevant variables such as social stress and social defeat. We will describe studies that utilize a novel animal model of drug abuse in which male monkeys live in social groups and have access to cocaine daily.
To relate the preclinical findings to a more global goal of translational research, it is important to point out several advantages of the animal models highlighted in this article. First, drugs can have different effects on brain function depending on whether they are administered by the investigator or self-administered by the animal
(31,
32). Because we are interested in studying addictive
behavior, drug self-administration has face validity as an animal model of a human condition. Second, in our studies of nonhuman primates we also examine complex social behaviors and stable individual behavioral characteristics (e.g., aggressive and affiliative behaviors in particular) that closely model human social interactions (see reference
33). In macaque monkeys, the formation of social hierarchies is determined by the outcome of physical confrontations (i.e., fights). The monkey that wins all of the fights in the social group is the most dominant, the monkey that wins all encounters except with the most dominant animal is the monkey ranked number 2, and so forth. This hierarchy is transitory and linear, such that if number 2 wins a battle with number 3 and number 3 is ranked above number 4, then number 2 is above number 4. We view the linear hierarchy that forms, with the dominant monkeys at the top and the subordinate animals at the bottom, as a continuum from environmental enrichment at one end to high levels of stress at the other. Socially derived stressors have high ethological validity with regard to the study of human drug abuse. Finally, nonhuman primates have many advantages over other animal species. There is abundant evidence that rodent and primate brains differ in the anatomy, physiology, and neurochemistry of brain dopamine systems
(34–
37). Furthermore, the nonhuman primate brain differs substantially from the rodent brain in terms of cocaine-induced changes in brain metabolism
(38,
39). Nonhuman primates can be studied in long-term experiments with cocaine self-administration (over years) that use within-subject designs, and they are capable of learning complex behaviors in order to obtain drugs. The use of PET imaging to examine neuroadaptations allows for longitudinal examination of brain changes due to environmental and/or pharmacological manipulations (e.g., references
40–42). The studies described in the following sections will focus on three phases of drug addiction: vulnerability, maintenance, and abstinence/relapse.
Relation of D2 Receptor Binding to Vulnerability to Cocaine Abuse
Perhaps one of the most challenging issues in drug abuse research involves understanding the etiology of addiction. Epidemiological studies suggest that approximately 17% of the people who use cocaine become dependent on the drug
(43,
44). Studies in humans can generate hypotheses about potential brain markers that may indicate a predisposition to become addicted. For example, Volkow et al.
(45) studied 23 non-drug-abusing male subjects and used the dopamine transport inhibitor methylphenidate as a tool to study stimulant abuse. First, each subject was scanned with the D2 receptor ligand [
11C]raclopride. On another day, they were administered 0.5 mg/kg of methylphenidate and asked to complete an analogue self-rating scale for pleasant drug effects. Approximately one-half (N=12) of the 23 subjects reported liking the dose of methylphenidate, while nine of them described it as “unpleasant” (two were indifferent to the drug effect). Subjects who found methylphenidate “pleasant” had significantly lower levels of D2 receptor binding than subjects who reported the drug as “unpleasant.” While these findings suggest an inverse relationship between D2 receptor availability and “vulnerability” to stimulant reinforcement, because of ethical concerns humans cannot be studied prior to drug exposure and then allowed to self-administer the drug for an extended period of time. Animal models, however, can be used to test hypotheses generated from studies of human drug abusers.
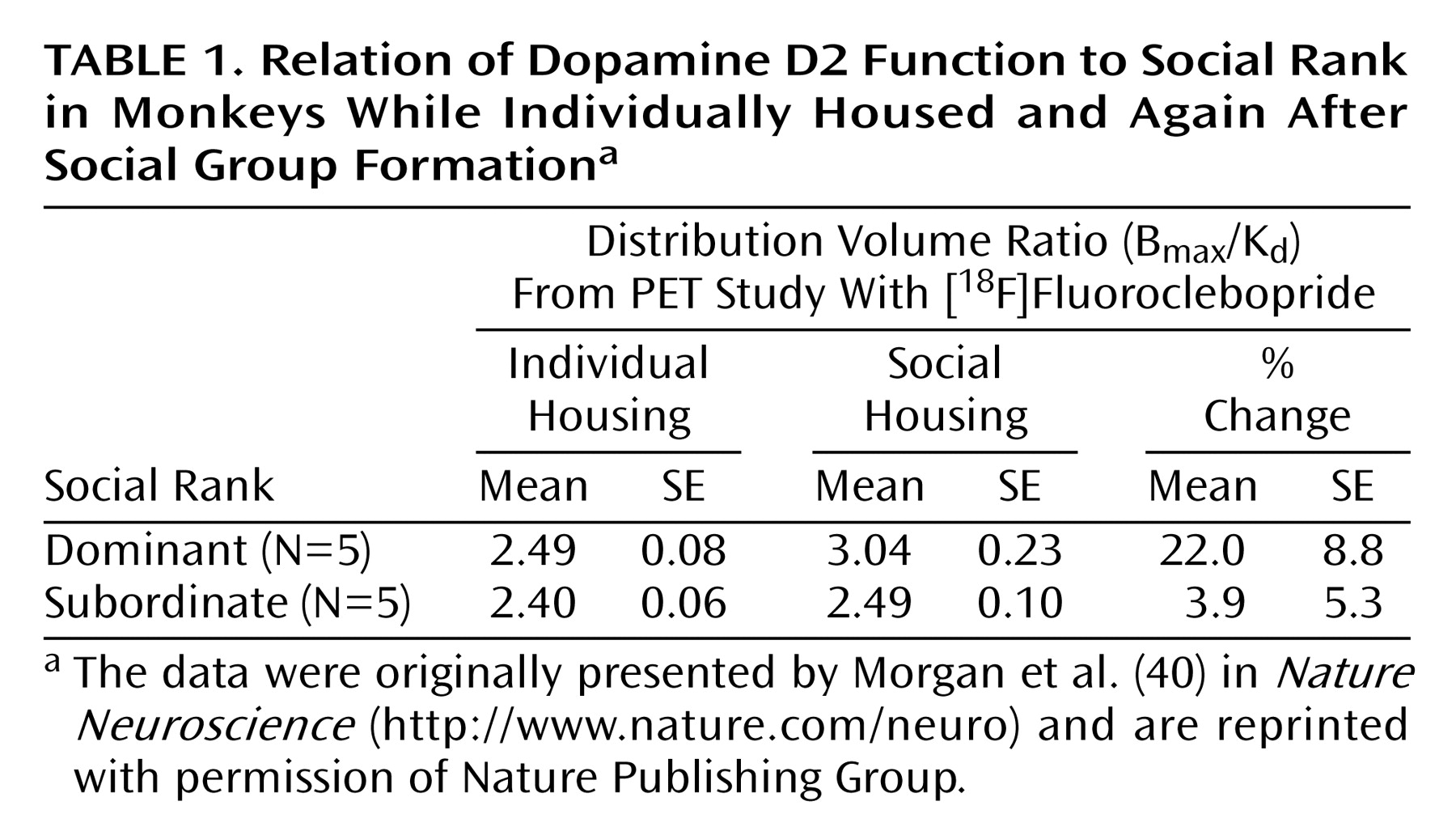
We explored further the relationship between D2 receptors and sensitivity to psychostimulants in a novel model of drug abuse in monkeys housed together
(40). Previous work from our group had shown a relationship between D2 receptor availability, as measured with PET, and the social rank of female monkeys
(46). Subordinate monkeys had significantly lower levels of D2 receptor binding than dominant monkeys. This relationship between social rank and D2 receptors generated an interesting series of questions related not only to drug abuse but also to trait theory. Our first question was whether D2 receptor availability influenced social rank. That is, were monkeys genetically predisposed to a particular position in the social hierarchy according to basal D2 receptor availability? To answer this question, we studied 20 individually housed male monkeys by using the D2 receptor ligand [
18F]fluoroclebopride
(47) before the monkeys were housed together. The level of D2 receptor binding determined during individual housing did not predict eventual social rank. That is, D2 availability was not a trait variable influencing dominance hierarchies
(40).
The next question was whether formation of the social hierarchies influenced D2 receptor availability. When these animals were rescanned after 3 months of social housing, there were significant differences between groups, with the subordinate monkeys having significantly lower D2 receptor binding than the dominant monkeys
(40). This finding replicated our earlier work with female monkeys
(46) and extended it to males. However, when we compared each monkey’s [
18F]fluoroclebopride distribution volume ratio when they were individually housed to the new ratio after social group formation, a profound effect was noted: the average distribution volume ratios for dominant monkeys increased by over 20%, while those for the subordinate animals were nearly unchanged from their original baselines (
Table 1). These results suggest that becoming the dominant monkey in the social group produced large changes in dopamine receptor function.
One hypothesis that could account for the observed changes is that being the dominant monkey is analogous to living in an enriched environment. Dominant animals have access to treats in the pen, they are groomed more often than subordinate monkeys, and they move about freely
(33). Enrichment could affect the PET signal (i.e., produce increases in the distribution volume ratio) by increasing D2 receptor densities and/or decreasing levels of extracellular dopamine in dominant monkeys. Studies using rodents have clearly shown that environmental enrichment can affect dopamine neurotransmission in an orderly and reliable fashion consistent with both potential mechanisms. For example, Bowling et al.
(48)) examined the effects of different rearing environments on dopamine synthesis and metabolism in rats. The group in the “enriched” condition lived together, with 12 or 13 rats per cage, and was exposed to novel toys. Another group lived under “impoverished” conditions consisting of individual housing without toys in the cage. In vitro studies indicated that the rats in the enriched condition had lower striatal concentrations of dopamine than the rats in the impoverished condition. In another study, Hall et al.
(49) used in vivo microdialysis to compare rats reared in isolation with socially reared rats, and they found that dopamine levels in the nucleus accumbens were higher in the isolation-reared rats than in the socially reared rats. Furthermore, D2 receptor densities were lower in the isolation-reared rats than in the socially reared rats
(49). These findings clearly show that environmental variables as seemingly subtle as housing conditions can produce profound changes in the functional status of dopamine systems. Overall, the findings in rodents are in the same direction as the effects we observed after formation of social groups by monkeys.
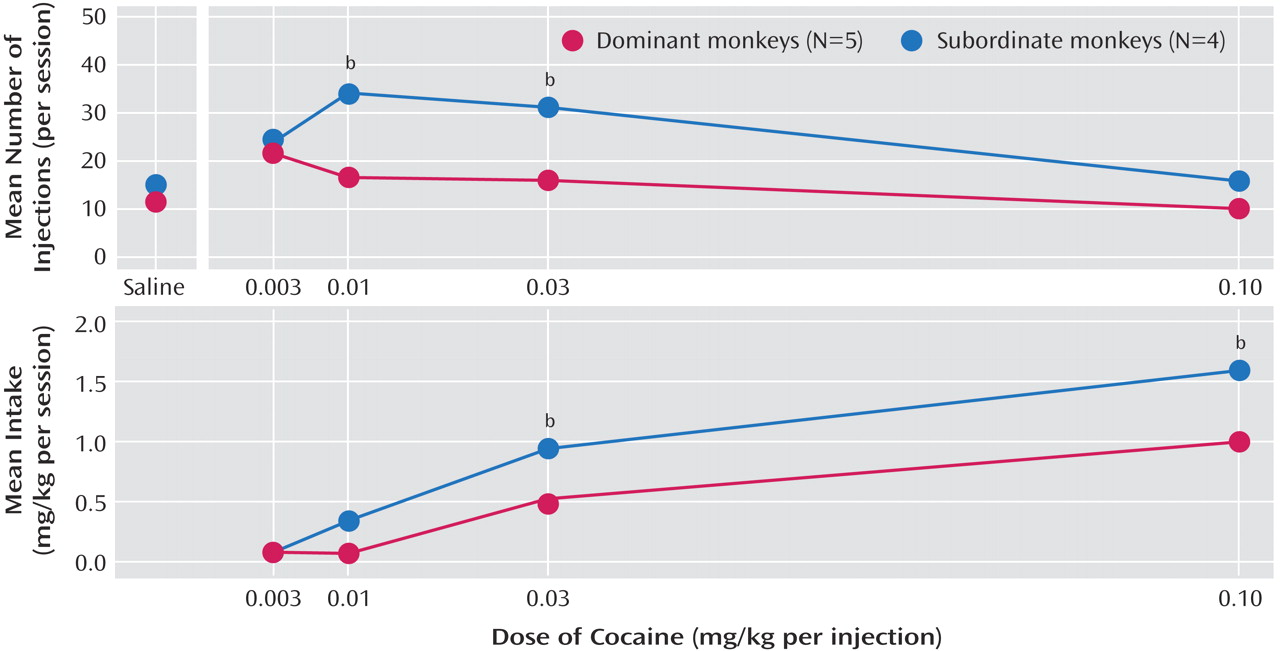
An important question is whether these environmentally induced brain changes have behavioral consequences. In particular, how do these variables influence vulnerability to drug abuse? If, as previously noted in humans, there is a relationship between D2 receptor availability and stimulant reinforcement, then there should be differences in drug reinforcement between dominant and subordinate monkeys and subordinate animals should be more sensitive to the effects of cocaine. This is, in fact, what we found
(40). When subsequently allowed access to cocaine, subordinate animals self-administered cocaine at higher rates and had larger intakes than those for dominant monkeys (
Figure 1). Overall, these findings confirmed an inverse relationship between D2 receptor availability and cocaine reinforcement and suggested that environmental variables could affect brain function and vulnerability to cocaine abuse.
Decrease of D2 Receptor Binding With Continued Cocaine Use
Human PET imaging studies have shown lower D2 receptor availability in cocaine abusers than in age-matched comparison subjects
(11). However, as already described, it is not clear whether the lower D2 receptor binding was a predisposing trait or a consequence of cocaine exposure (see reference
11). Understanding how long-term cocaine exposure affects dopamine receptor function could ultimately lead to better treatment strategies. One hypothesis is that D2 receptor down-regulation occurs as an adaptation to the chronic elevation in extracellular dopamine due to chronic blockade of dopamine uptake. A strength of using animal subjects is the ability to study drug-naive subjects and to observe changes in receptor availability with exposure to a drug by means of a within-subjects, longitudinal design (see reference
50, for example).
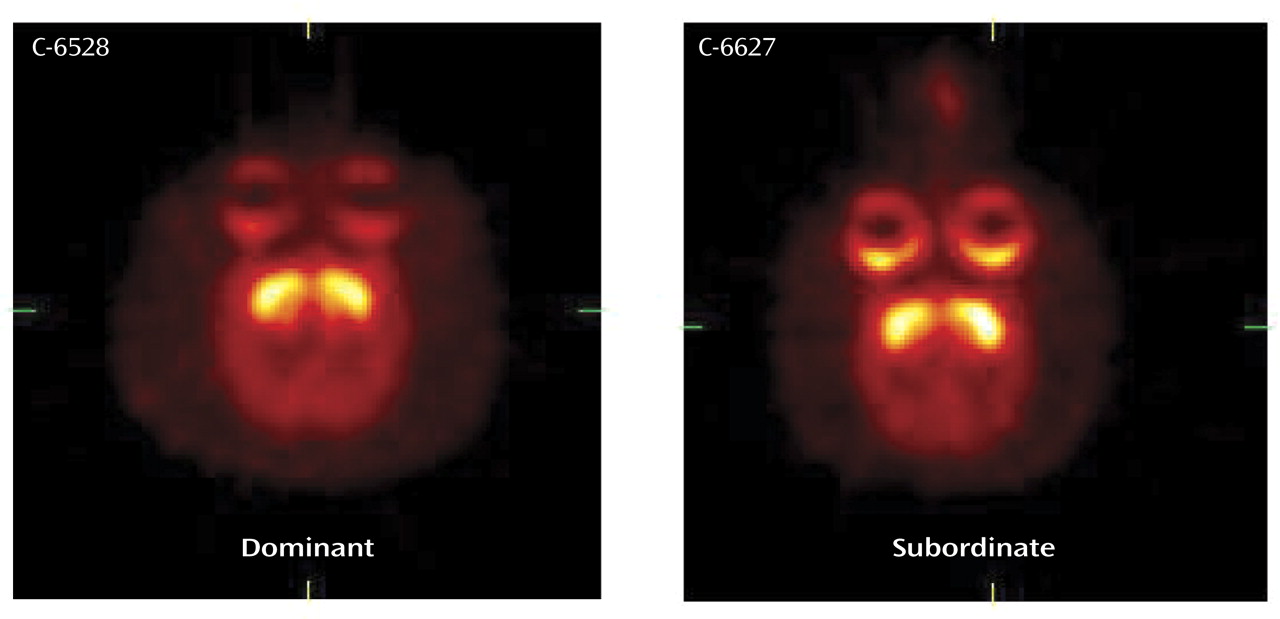
We recently extended our studies of socially housed monkeys to examine the effects of chronic cocaine exposure in dominant and subordinate monkeys
(51). Whereas dominant monkeys were initially protected from the reinforcing effects of cocaine by elevated D2 availability, chronic exposure to self-administered cocaine resulted in D2 measures that were no longer different from those of subordinate monkeys (
Figure 2). These findings suggested that exposure to cocaine attenuated or reversed the powerful effects of environmental context on dopamine receptor availability. Studies utilizing in vitro receptor autoradiography of D2 receptors have consistently shown lower receptor densities in monkeys with long-term histories of cocaine self-administration; many of these effects were directly related to dose and duration of exposure
(52–
54).
A central issue for this phase of addiction is one that was raised for the first phase, namely individual differences. For example, one issue to consider in addition to the dose of a drug taken in the lifetime of the individual is the pattern and duration of drug use. Volkow et al.
(11) noted that there was not a relationship between D2 receptor binding and the dose of cocaine used, but there was a significant correlation between D2 binding and duration of cocaine use. The effects of duration and cocaine intake can be addressed with animal models in which cocaine availability is the primary independent variable. The question could be phrased as, Does administering a large amount of cocaine over a short period of time produce greater long-term effects on dopamine receptor function than does administering moderate doses over longer periods? In a study not utilizing cocaine self-administration, in vitro receptor autoradiography was used to assess the consequences of chronic cocaine use and abstinence on D2 receptor levels
(55). Monkeys were treated four times per day with cocaine for 2 consecutive weeks, followed by a 2-week withdrawal period. The investigators found no differences between cocaine-treated and control monkeys in D2 receptor densities in the caudate nucleus, prefrontal cortex, substantia nigra, and nucleus accumbens. However, this amount of cocaine administered has since been shown to produce robust decreases in D2 receptor densities when
self-administered over longer periods of time (see, for instance, references
52 and
53). Such findings suggest that the duration of exposure is as important as the actual dose of drug administered and reinforce the importance of using contingent drug administration in animal models of addiction. Future studies using in vivo imaging techniques will better address this extremely important question.
As we discussed for the vulnerability phase, an important question is whether there are functional consequences to cocaine-induced changes in D2 receptor binding. Of particular interest are issues related to cognitive function, decision making, and choice behavior. For example, Grant et al.
(56) compared a group of drug abusers (opiate and/or stimulant) and non-drug-abusing comparison subjects on a series of neuropsychological tests to examine the long-term consequences of drug use on decision making. In one set of studies, subjects were exposed to the “gambling task”
(57), which has strong face validity for evaluating cognitive deficits
(56). The task involved four different decks of cards that differed along three dimensions: immediate gain, long-term expected gain, and schedule of penalties. Two of the four decks had smaller gains and punishers but higher overall “yields”; the comparison subjects chose from those decks most often. In contrast, the majority of the drug abusers chose from the low-yield decks, indicating poor decision making. To control for possible differences in IQ, Grant et al.
(56) also used the Wisconsin Card Sorting Test and found no differences between the groups. The authors concluded that impairments in decision making could certainly account for continued use of drugs in the presence of adverse social and personal consequences.
Studies with cocaine abusers cannot clarify whether such cognitive impairments are a consequence of excessive cocaine use or reflect preexisting decrements that predispose certain individuals to drug abuse. Animal studies can address this issue. Using our model with group-housed monkeys, we studied choice behavior involving cocaine and a nondrug reinforcer—banana-flavored pellets
(27). Subordinate monkeys were more sensitive to the reinforcing effects of cocaine than were dominant monkeys. That is, subordinate monkeys chose cocaine over food at a lower cocaine dose than that for dominant monkeys. Such findings suggest that environmental context can substantially influence the preference to choose drug over nondrug alternatives even when D2 binding does not differ between groups.
Variable Recovery of D2 Receptor Binding During Cocaine Abstinence
The final phase of drug addiction to be discussed is abstinence and the variables that influence relapse. As with vulnerability and maintenance, we will focus on dopamine D2 receptor availability. From a clinician’s viewpoint, abstinence is the critical phase because the population seeking professional guidance and treatment are in this phase; it is worth pointing out that this is the phase of drug addiction about which we know and understand the least. The preceding issues related to vulnerability (predisposition) and maintenance (i.e., changes in brain function due to chronic drug exposure) are critical in understanding brain changes associated with abstinence. However, understanding these variables may provide only a glimpse of the complexity involved in brain changes during abstinence. Perhaps Childress et al. stated it best: “This search [for a cocaine medication] has been complicated by the heterogenous target: drug desire that emerges during cocaine cessation…may well have a different brain substrate than desire induced by cocaine itself…and the cues that signal it”
(58, p. 11).
Over a decade ago, Volkow and colleagues
(59) reported significantly lower D2 receptor binding in cocaine abusers abstinent from cocaine for up to 4 months. These reductions in D2 receptor binding were associated with decreased glucose utilization in the orbitofrontal cortex and with self-ratings of dysphoria
(11). Thus, there was an association between dopamine receptor availability and negative subjective effects during abstinence. The study ended 4 months after the start of abstinence because all subjects had relapsed. Animal models, on the other hand, can provide valuable insight into the neuropharmacology of abstinence and relapse because abstinence can be studied for years.
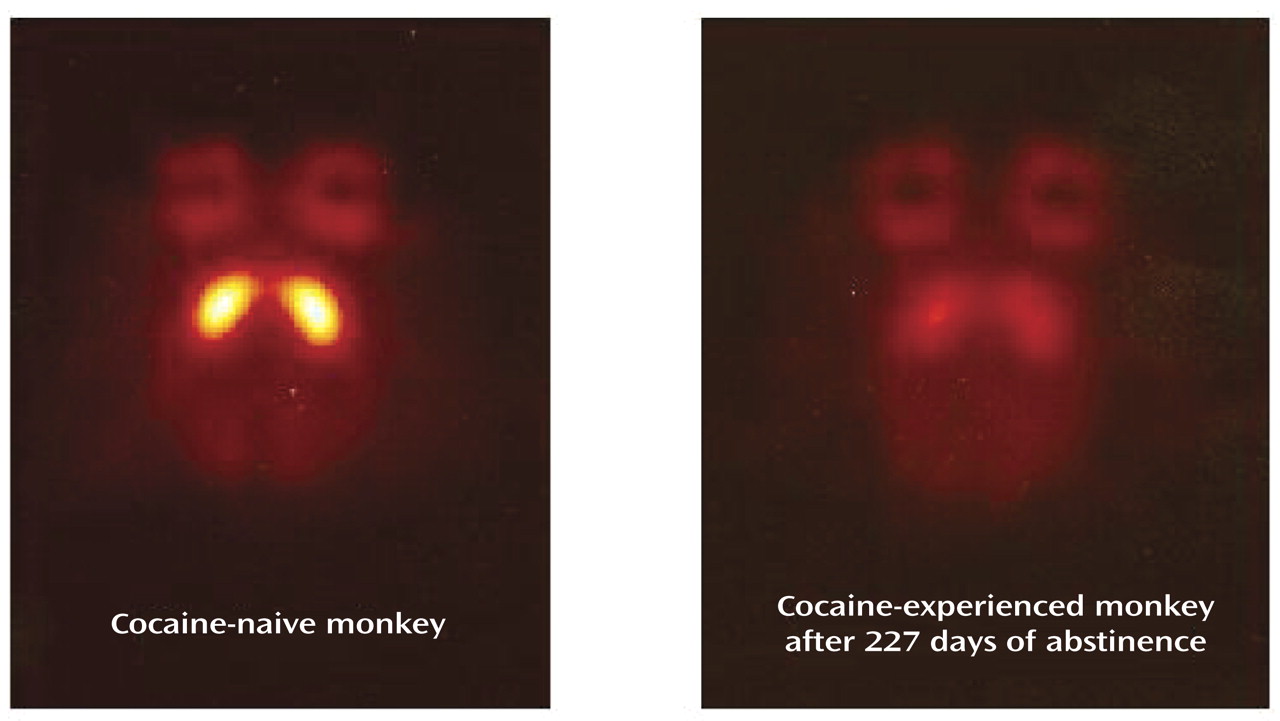
Our first efforts to study D2 receptor function during abstinence involved using PET imaging to compare D2 receptor binding in monkeys with extensive histories of cocaine self-administration and cocaine-naive comparison monkeys (i.e., a group design). For example, the first monkey we studied had self-administered cocaine for over 3 years and had a lifetime cocaine intake of approximately 20 g. PET scans were conducted at various time points during abstinence. Differences in [
18F]fluoroclebopride binding between this cocaine-experienced monkey and a cocaine-naive monkey were apparent at all time points and, as shown in
Figure 3, did not dissipate even after 7 months of abstinence. The image from a comparison monkey shows excellent uptake of [
18F]fluoroclebopride into D2-rich regions of the basal ganglia. In the abstinent cocaine-experienced monkey, D2 receptor binding was more than 20% lower. While it is possible that the lower D2 signal in the cocaine-experienced monkey was due to elevated levels of dopamine, it is unlikely that such an effect would persist for 7 months. The other possibility is that chronic cocaine exposure resulted in persistent reductions in D2 receptor densities. This hypothesis has been confirmed by means of in vitro receptor autoradiography
(52,
53; see reference
54 for further discussion).
It is necessary to comment on animal models and “craving.” As defined by Hommer, craving “is a term derived from popular psychology that is used to describe one of the mental states—namely, the intense desire for a certain object or experience”
(60, p. 187). Assessing a mental state in an animal is not a realistic undertaking. However, we can measure behavior and hypothesize about mental states. Describing the multitude of animal models of craving is beyond the scope of this review. In brief, the strategies can involve persistence of drug seeking in the presence of stimuli that signal no drug is available
(61), escalation of drug seeking with changes in drug availability
(62), responding in the presence of conditioned stimuli
(63), or a return of responding that had been previously extinguished (i.e., reinstatement)
(64).
The reinstatement procedure has been widely used as a model of the ability of priming injections of drugs, drug-related cues, and stress to precipitate relapse into drug taking (reviewed in reference
65). Although the predictive and construct validity of the procedure remain to be firmly established
(66), the ability of noncontingent administration of cocaine to reinstate extinguished responding previously maintained by cocaine has been well established
(65). Furthermore, it has been demonstrated that drugs that directly or indirectly activate the dopamine system can reinstate cocaine seeking under a food-drug choice procedure
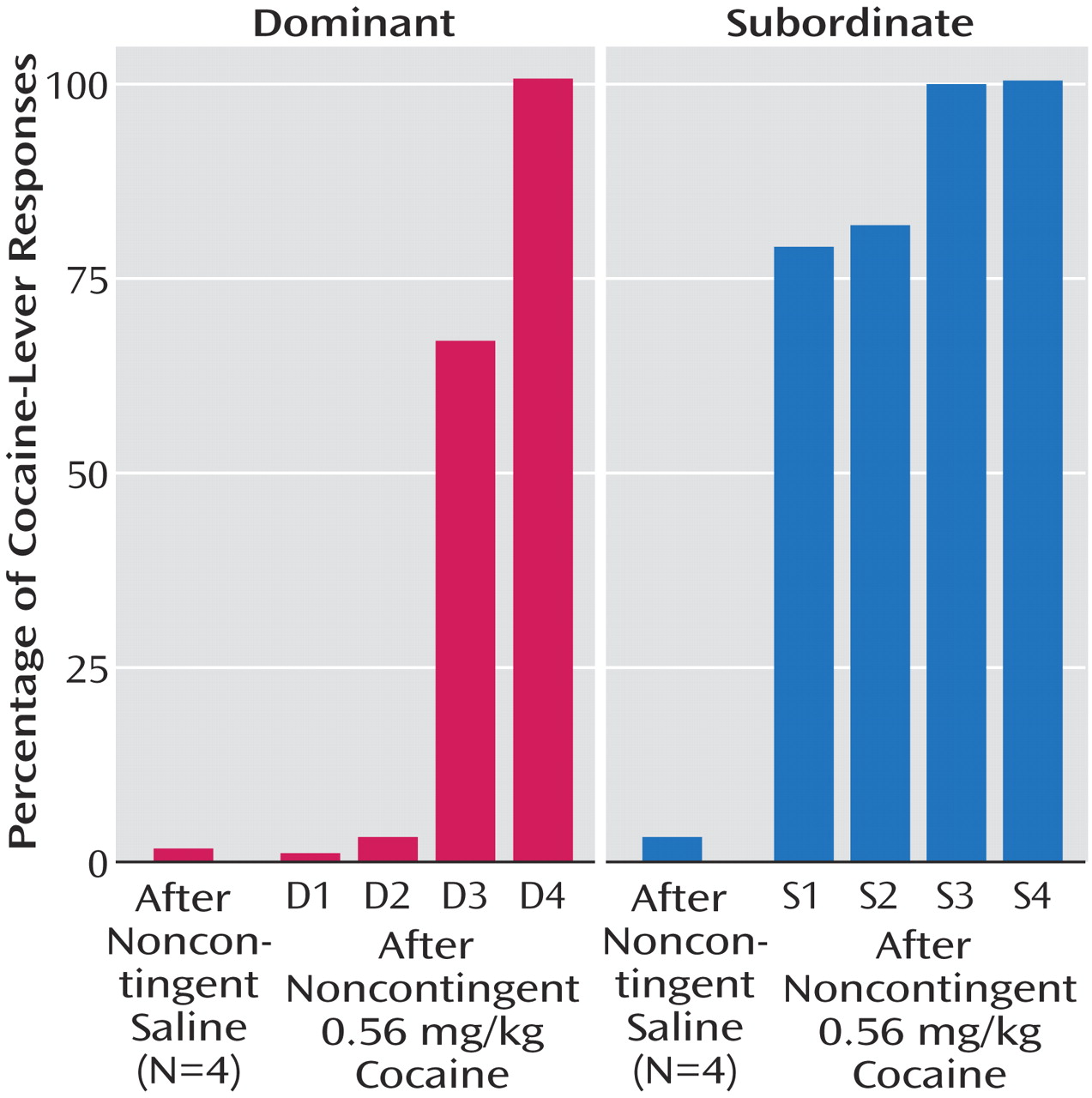
(67). Thus, the reinstatement procedure allowed us another means to assess potential differences in dopamine receptor function between dominant and subordinate monkeys. Socially housed monkeys were allowed to self-administer cocaine under conditions in which they also could choose food reinforcement. Under these conditions, as the cocaine dose increased, the percentage of total responses involving the cocaine-associated lever increased
(27). When saline was substituted for cocaine, the monkeys shifted their responses to the food-associated lever (
Figure 4). We next examined the ability of noncontingent cocaine to reinstate responses on the cocaine-associated lever, although these responses continued to produce saline injections. Noncontingent cocaine produced dose-related increases in responses on the cocaine-associated lever at doses up to 0.30 mg/kg in all monkeys (data not shown). However, dominant and subordinate monkeys differed in their responses to a higher cocaine dose (0.56 mg/kg). All four subordinate monkeys responded primarily on the injection lever following this dose of cocaine (
Figure 4). Greater variability was observed in the dominant monkeys’ responses to this dose. These data are consistent with our earlier results demonstrating that subordinate monkeys are, on average, more sensitive to the reinforcing strength of cocaine and/or less sensitive to the aversive effects of cocaine than are dominant monkeys.
Conclusions
In this review we have attempted to briefly highlight the use of animal models and brain imaging procedures (PET and in vitro receptor autoradiography) to convey a better understanding of the neuropharmacology of drug addiction. The focus on dopamine D2 receptors can be viewed as a strength as well as a limitation. The strength of this focus is the understanding of how the level of this receptor superfamily serves as a trait influencing vulnerability to drug abuse and how it serves as a potential state variable showing malleability as a result of environmental or pharmacological manipulations. The weakness of this focus on D2 receptors is the fact that drug addiction is not mediated by one receptor subtype. Future studies must examine how other neurotransmitter and neurohormone systems change in concert with brain dopamine systems, as well as the development of novel radioligands for specific receptor subtypes (e.g., D3 receptors). In addition, increased anatomical selectivity with higher-resolution PET cameras will better address the contribution of particular brain circuitry to behavioral effects.
The use of animal models to study human disease is an extremely important tool. We have described models that could be characterized as predictive, isomorphic, and homologous of the human condition. In particular, the use of socially housed nonhuman primates to better understand the neuropharmacology and the behavioral and social consequences of drug abuse and to evaluate potential treatment strategies has unequivocal potential. It is our hope that clinicians reading this review will gain a better understanding of the research questions being asked and the potential importance of the results for the development of therapies (behavioral and pharmacological) for drug addiction.
Finally, this review has provided evidence that the environment can profoundly affect drug abuse vulnerability, maintenance, and relapse. Environmental stress and enrichment can influence brain function and vulnerability to drug abuse. Furthermore, even following long-term drug use, environmental variables can affect the behavioral effects of cocaine. The research described in this review represents the growing number of studies documenting the benefits of environmental enrichment, irrespective of genetic predispositions to abuse drugs. Such preclinical findings suggest that outcome measures will be enhanced not only by taking individuals out of a stressful environment but also by providing alternative reinforcers—whether these are better living conditions, jobs, or other activities
(68).