Mismatch negativity is an event-related potential component that is automatically evoked when a sequence of repetitive standard auditory stimuli is interrupted by “oddball” or “deviant” stimuli (e.g., infrequent stimuli that differ in duration from the more frequently presented or “standard” stimuli). Mismatch negativity has several advantages for neuropsychiatric studies, including the exploration of the neural substrates of schizophrenia and its treatment
(1). In addition, mismatch negativity is increasingly used in assessments of “difficult-to-test” populations (e.g., infants, comatose subjects, and those with brain injuries) and offers promise for characterizing the integrity of sensory network dysfunction in patients with schizophrenia
(1).
The duration-deviant mismatch negativity paradigm differentiates patients with schizophrenia from normal comparison subjects with large effect sizes
(2) and diagnostic specificity relative to other psychiatric disorders
(3). We have reported that mismatch negativity deficits in schizophrenia are highly associated with patients’ impairments in everyday functioning
(2) and have proposed that mismatch negativity (and other largely “preattentional” measures) be used as targets for assessing the efficacy of medications aimed at improving the cognitive and functional deficits of patients with schizophrenia
(1).
The high reliability of duration mismatch negativity in normal subjects has led to the view that mismatch negativity may have potential for a variety of clinical applications
(4). Neither short- nor long-term reliability of mismatch negativity has been definitively established in patients with schizophrenia. If mismatch negativity deficits are stable in patients with chronic schizophrenia, then mismatch negativity may show promise for characterizing the neural substrates of the sensory processing and “real-world” functional deficits in longitudinal studies
(2). The aims of the present study were to 1) determine if mismatch negativity deficits are stable in patients with chronic schizophrenia over a 1–2-year period and 2) examine the longitudinal relationship of mismatch negativity to functional status.
Method
Ten normal comparison subjects and 10 patients with schizophrenia participated in the study. The patients with schizophrenia underwent two identical recording sessions separated by at least 1 year (mean retest interval=578 days, SD=39.3). All screening and assessment procedures were performed in both sessions for the patients with schizophrenia according to our reported methods
(2). All participants were assessed as being capable of providing informed consent. After complete description of the study to the subjects, we obtained written informed consent using methods approved by the University of California, San Diego, Institutional Review Board (IRB numbers 030510 and 040564).
All subjects received a urine toxicology screen, the Structured Clinical Interview for DSM-IV, the Scale for the Assessment of Negative Symptoms (SANS)
(5), the Scale for the Assessment of Positive Symptoms (SAPS)
(6), and the Global Assessment of Functioning Scale (GAF) (DSM-IV-TR, p. 34). The majority of patients were prescribed second-generation antipsychotic medications (N=8); one patient reported not taking antipsychotic medications for at least 7 days before testing. Normal subjects were recruited and screened according to reported methods
(2). No statistically significant differences in hearing thresholds between groups were observed.
Subjects were presented with binaural stimulation (1-kHz computer-generated square-wave stimuli, 85 dB[A] SPL, 1 msec rise/fall) with a fixed stimulus onset-to-onset asynchrony of 500 msec. Ninety percent standard (50 msec) and 10% deviant (100 msec) duration stimuli were presented to subjects in pseudorandom order while subjects watched a silent video.
EEG was acquired with 34 Ag/AgCl electrodes arranged in an EasyCap (Falk Minow Services, Herrsching-Breitbrunn, Germany) with all impedances below 4 kΩ following our reported methods
(2). Electrodes placed at the tip of the nose and at FPz served as the reference and ground, respectively. Additional electrodes were used for monitoring blinks and eye movements. Signals were digitized at a rate of 1 kHz with system acquisition filter settings at 0.5–100 Hz and were processed according to our reported methods
(2). Mismatch negativity waveforms were generated by subtracting event-related potential waveforms in response to standard tones from the event-related potentials generated in response to the deviant tones. The mismatch negativity amplitude was measured as the mean voltage from 135 to 205 msec.
Results
None of the patients with schizophrenia experienced significant changes in their community living situation, clinical status, or medication status in the test-retest interval. Similarly, clinical symptoms (SAPS: r=0.81, N=10, p<0.01; SANS: r=0.84, N=10, p<0.01) and functional status (GAF: r=0.62, N=10, p<0.05) were stable over time.
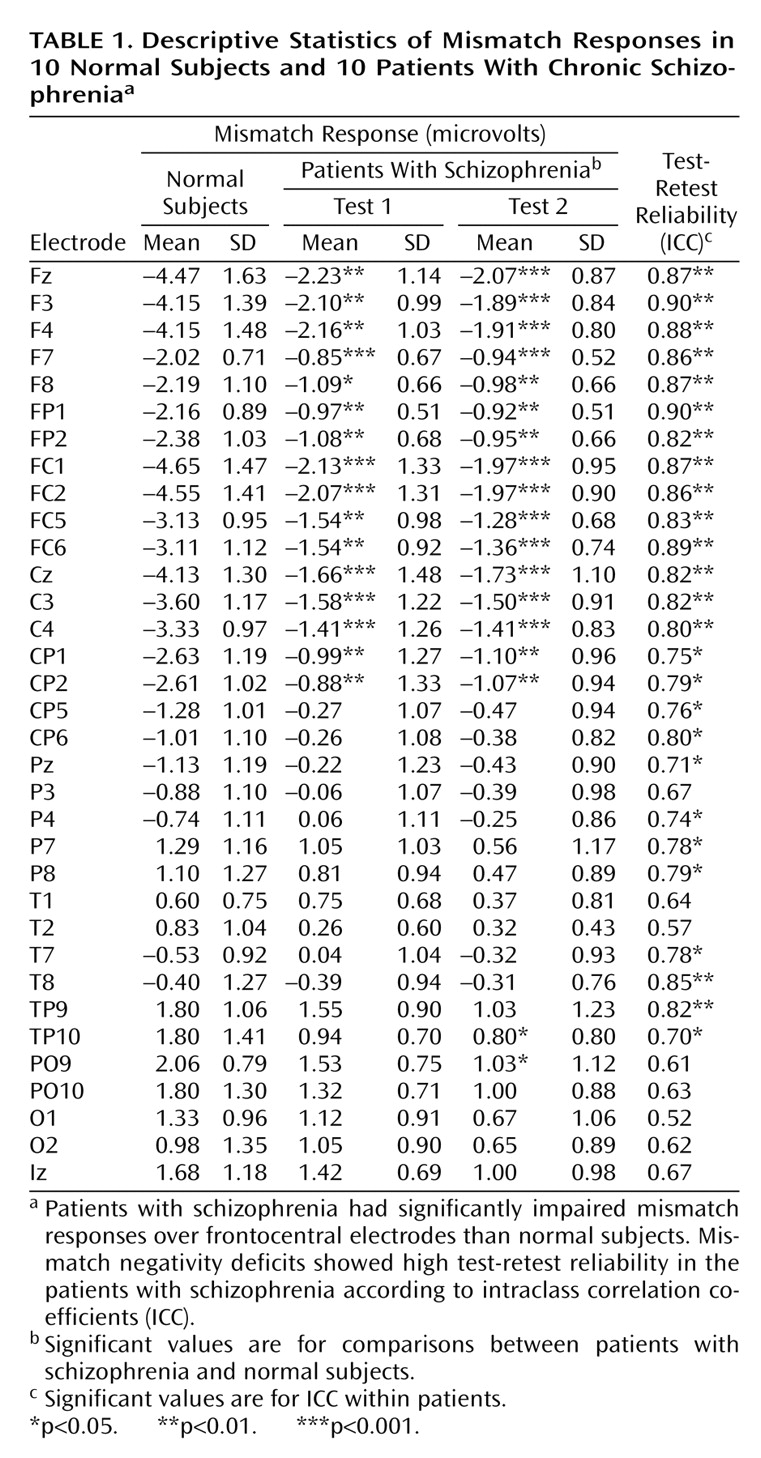
Patients with schizophrenia had mismatch negativity deficits at both the first (F=7.92, df=33, 594, p<0.001) and second (F=11.05, df=33, 594, p<0.001) recording sessions at frontocentral but not posterior electrodes (
Table 1). Within the patients with schizophrenia, no significant session effect or electrode-by-session interaction was observed, demonstrating overall stability across test sessions. In addition, intraclass test-retest correlation coefficients were highly significant at frontocentral electrodes (
Table 1).
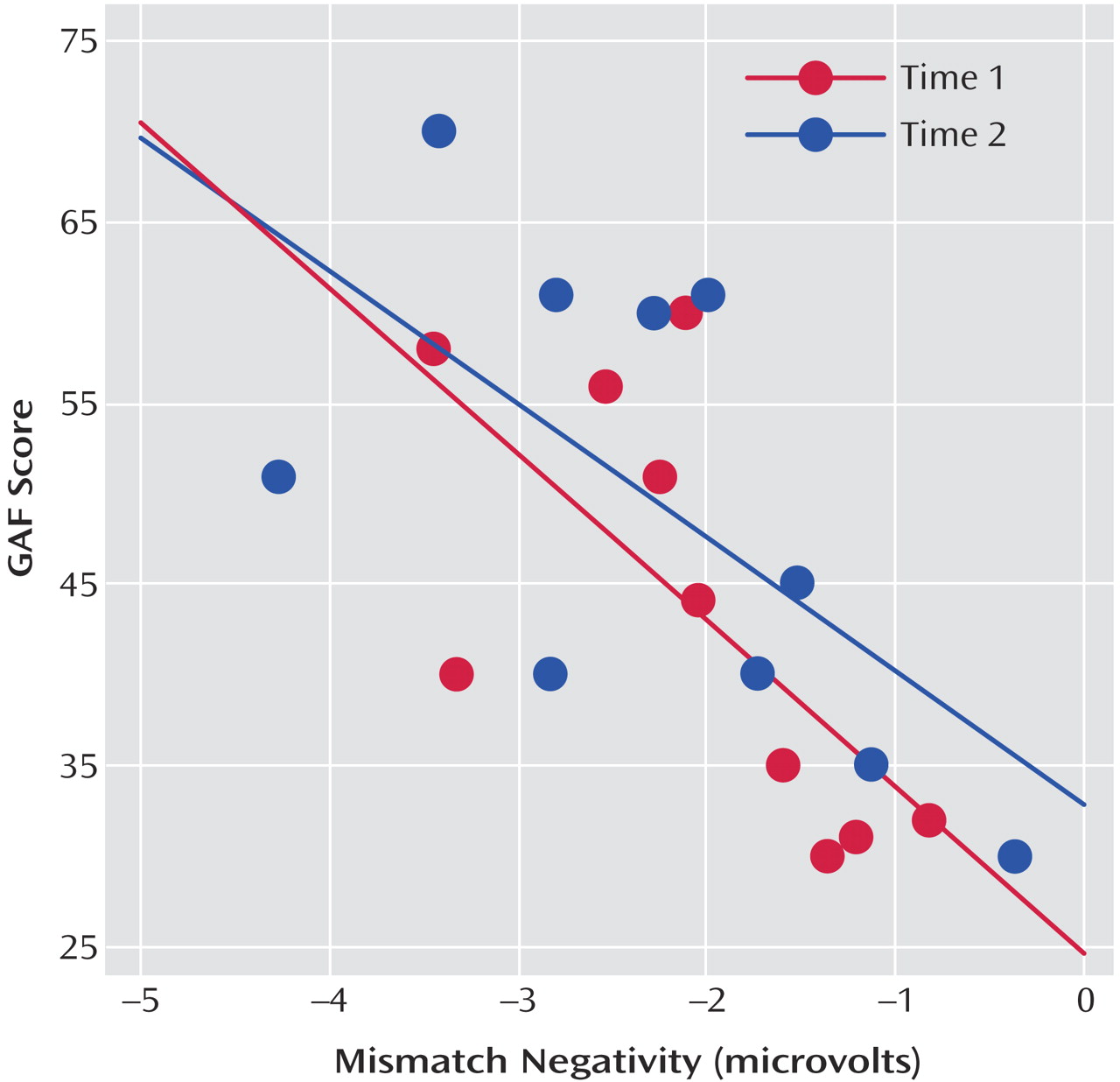
Mismatch negativity was significantly associated with GAF ratings at both the first (r=–0.63, N=10, p<0.05) and second (r=–0.68, N=10, p<0.05) sessions at frontocentral electrodes (
Figure 1). In contrast, mismatch negativity was not significantly correlated with positive or negative symptoms at either test session or when averaged across both sessions (all p>0.10).
Discussion
The results of the present study represent the first report to our knowledge of individual and group stability of mismatch negativity over a long test-retest interval in patients with chronic schizophrenia. Indeed, the high reliability of duration mismatch negativity previously reported in normal subjects (e.g., reference
4) is equivalent to the present results in patients with schizophrenia. In addition, this study now extends the previously reported relationship between mismatch negativity and functional status from cross-sectional to longitudinal cohorts
(2).
Clinically unaffected relatives of patients with schizophrenia
(7) and patients with chronic schizophrenia
(2,
3,
8) have been reported to have mismatch negativity deficits, but first-episode patients appear to have normal mismatch negativity
(8). If a progression of mismatch negativity deficits is observed in longitudinal studies, then mismatch negativity may be useful for tracking progressive neural substrate dysfunction and related functional impairments across the course of the illness. In the present study, we did not see statistically significant evidence of a progression of mismatch negativity deficits. Examination of means at frontocentral electrodes, however, shows slight (i.e., 0.2 volt or 5%) diminution of mismatch negativity amplitudes over the test-retest interval. Thus, to detect mismatch negativity deterioration over time, one should either start with first-episode patients or perhaps use a longer test-retest interval where a 5% change every 1–2 years would lead to a significant cumulative effect.
In summary, a crucial question is whether mismatch negativity deficits progress from the prodrome, to illness onset, and through the chronic course of illness in patients with schizophrenia. Additional studies with greater power are needed to clarify the longitudinal course of mismatch negativity deficits and to determine if these deficits can serve as biomarkers of neurodegeneration in patients with schizophrenia. The current results also demonstrate that a relationship exists between mismatch negativity deficits and poor functioning both cross-sectionally and longitudinally in patients with schizophrenia. Thus, the high test-retest reliability and association of mismatch negativity with functional outcome suggest that mismatch negativity may be a useful biomarker for assessing medication response in trials designed to improve the cognitive and functional impairments of patients with schizophrenia.