Patients
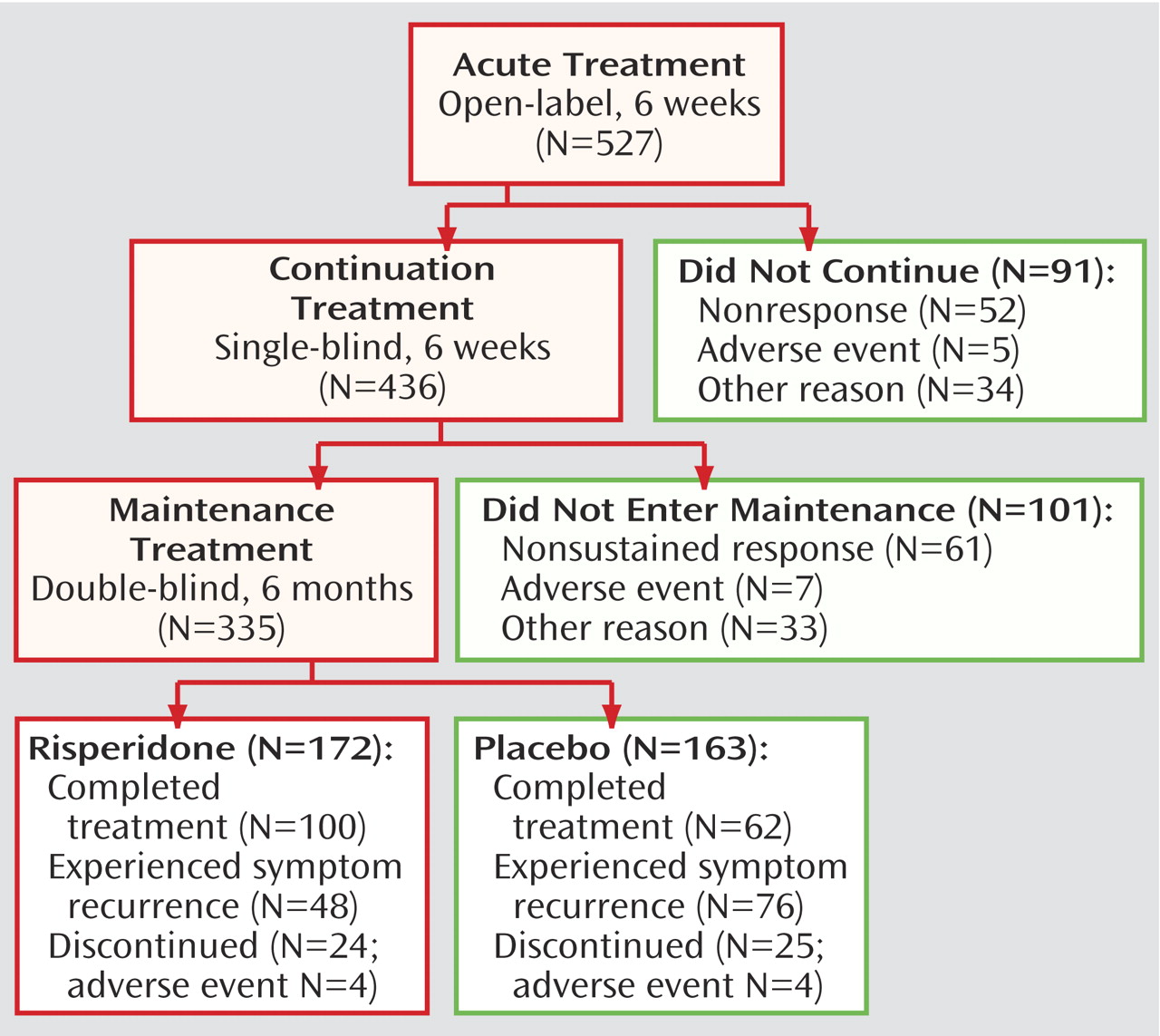
A total of 575 patients were screened and of these, 527 patients were enrolled in the 6-week, open-label, acute treatment phase. Overall, 436 patients continued single-blind treatment for an additional 6 weeks, and 335 were randomly assigned to 6 months of double-blind maintenance treatment (
Figure 1). Patient demographics are shown in
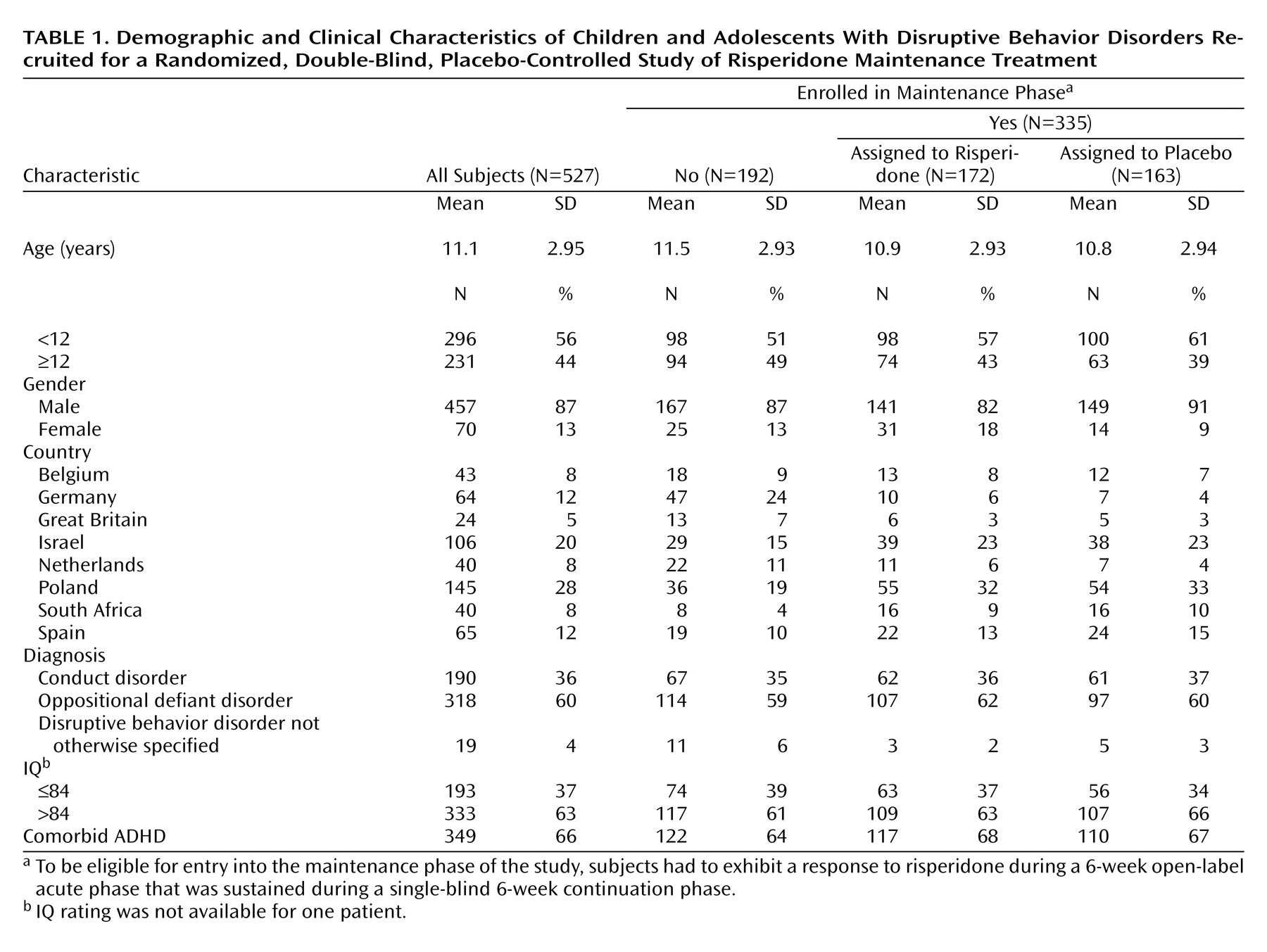
Table 1. The majority of patients were Caucasian (87%, N=459) and of normal (IQ>84) intellect (63%, N=333); a significant proportion (44%, N=231) were adolescents. Most (66%, N=349) had comorbid ADHD.
The average Nisonger Child Behavior Rating Form conduct problem subscale score of 35.2 (SD=6.29) at screening indicates that patients had severe symptoms. The symptom rated most often with the visual analogue scale as the most troublesome symptom was categorized as either aggression (46%) or oppositional defiant behavior (43%). Mean Children’s Global Assessment Scale score at screening was 47.0 (SD=9.9), corresponding to a moderate interference in most social areas or severe impairment in one area.
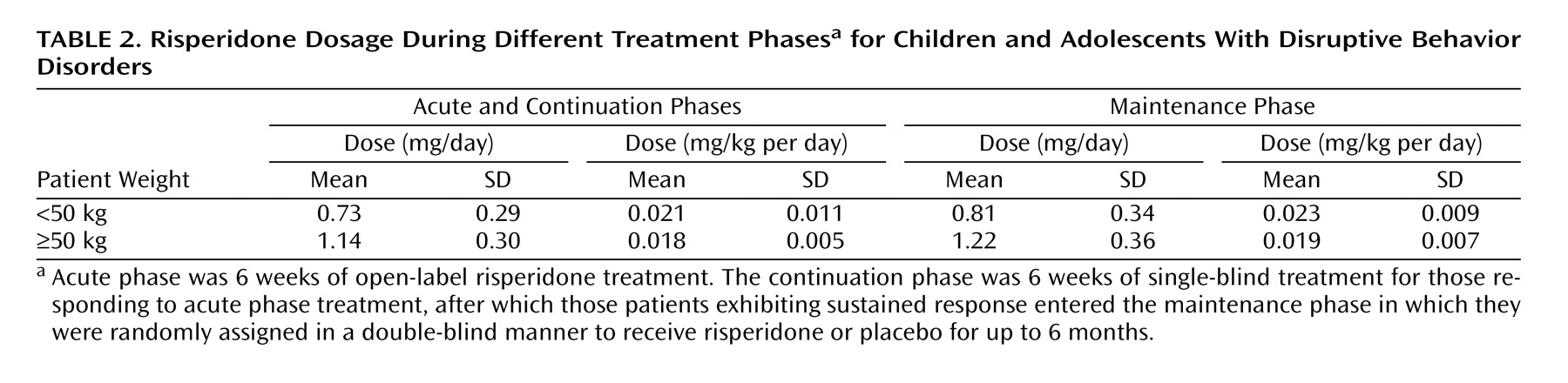
Treatment was discontinued before entering the double-blind maintenance phase by 192 (36%) patients, with most discontinuations due to patients not meeting the criteria for either initial response (10%, N=52) or sustained response (12%, N=61). Other reasons for discontinuation during the acute and continuation phases were adverse events (N=12), patient lost to follow-up (N=20), patient withdrawal of consent (N=15), patient noncompliance (N=14), patient ineligibility for study continuation (N=4), and other reasons (N=14). Double-blind maintenance treatment was completed by 162 of the patients who started this phase (48%), with most discontinuations due to meeting symptom recurrence criteria. Mean risperidone dosage was approximately 0.02 mg/kg per day (
Table 2). Prior therapy was used by 351 (67%) of patients enrolled into the acute treatment phase, most commonly psychostimulants (39%), antipsychotics (26%), and anticonvulsants (7%). During acute and continuation treatment phases, 59% of all patients received concomitant medications (24% psychostimulants, 16% analgesics). During the maintenance phase, concomitant medications were taken by 55% of risperidone-treated patients (21% psychostimulants, analgesics 15%) and 50% of placebo-treated patients (22% psychostimulants, 12% analgesics).
Primary Efficacy
At the end of the maintenance phase, there was a significant difference in rate of symptom recurrence, based on efficacy scales and investigator impression, between those assigned to risperidone (27.3%, N=47) and those assigned to placebo (42.3%, N=69) (c
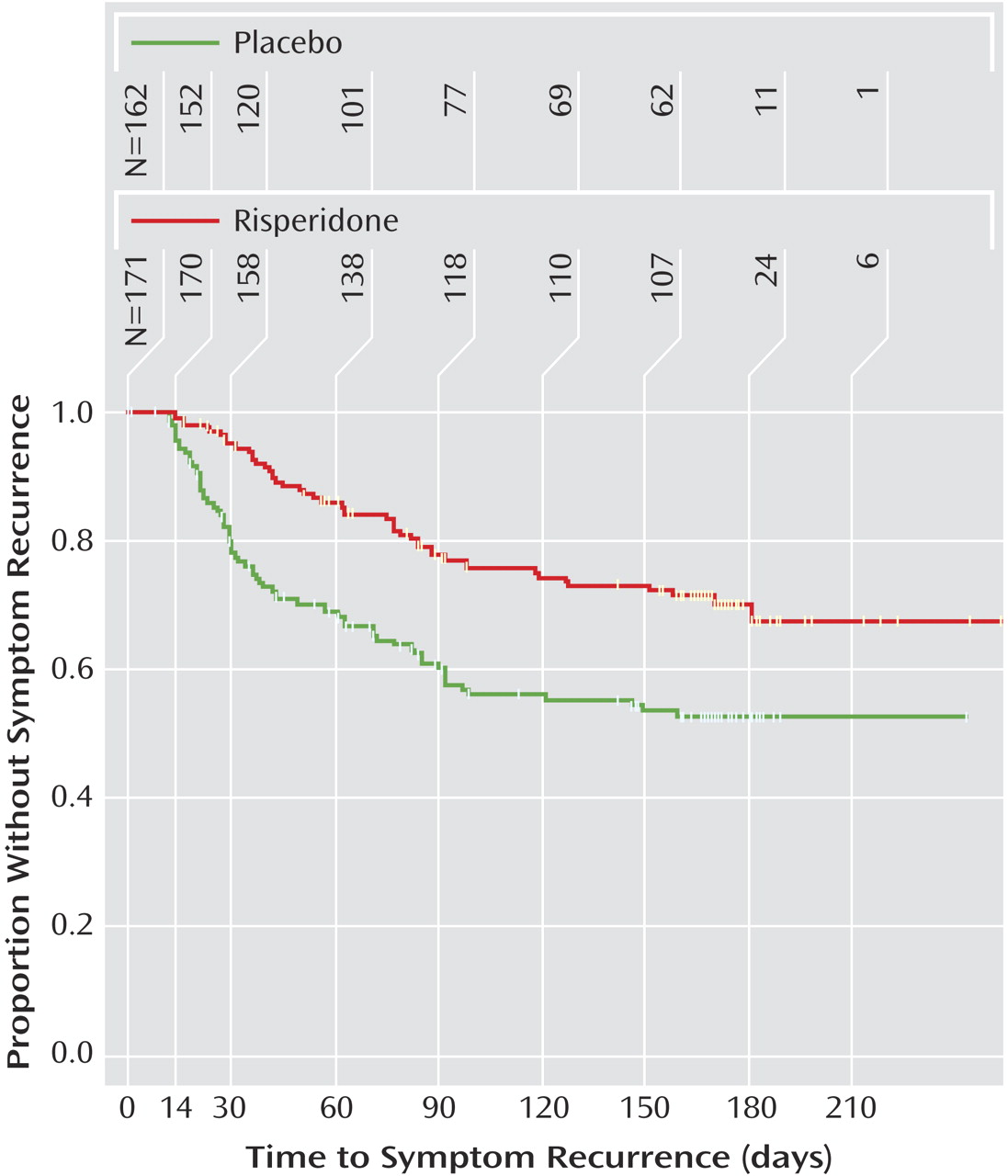
2=10.04, df=1, p=0.002). Time to symptom recurrence was significantly shorter with placebo compared with maintenance risperidone (c
2=18.45, df=1, p<0.001) (
Figure 2). Symptom recurrence occurred in 25% of patients after 119 days with risperidone and 37 days with placebo. Six-month Kaplan-Meier symptom recurrence estimates were 29.7% for risperidone and 47.1% for placebo. The instantaneous risk-to-symptom recurrence (hazard ratio) was 2.24 (95% CI=1.54–3.28) times higher after switching to placebo compared with continuing risperidone. Since less than 50% of patients met the criteria for symptom recurrence, the median time to symptom recurrence was not estimable. For patients who discontinued due to symptom recurrence, the range of time to symptom recurrence was 12–159 days among those receiving placebo and 14–181 days for those receiving risperidone. Risk for symptom recurrence was not affected by age, gender, diagnosis, or baseline disruptive behavior disorder severity.
Secondary Efficacy
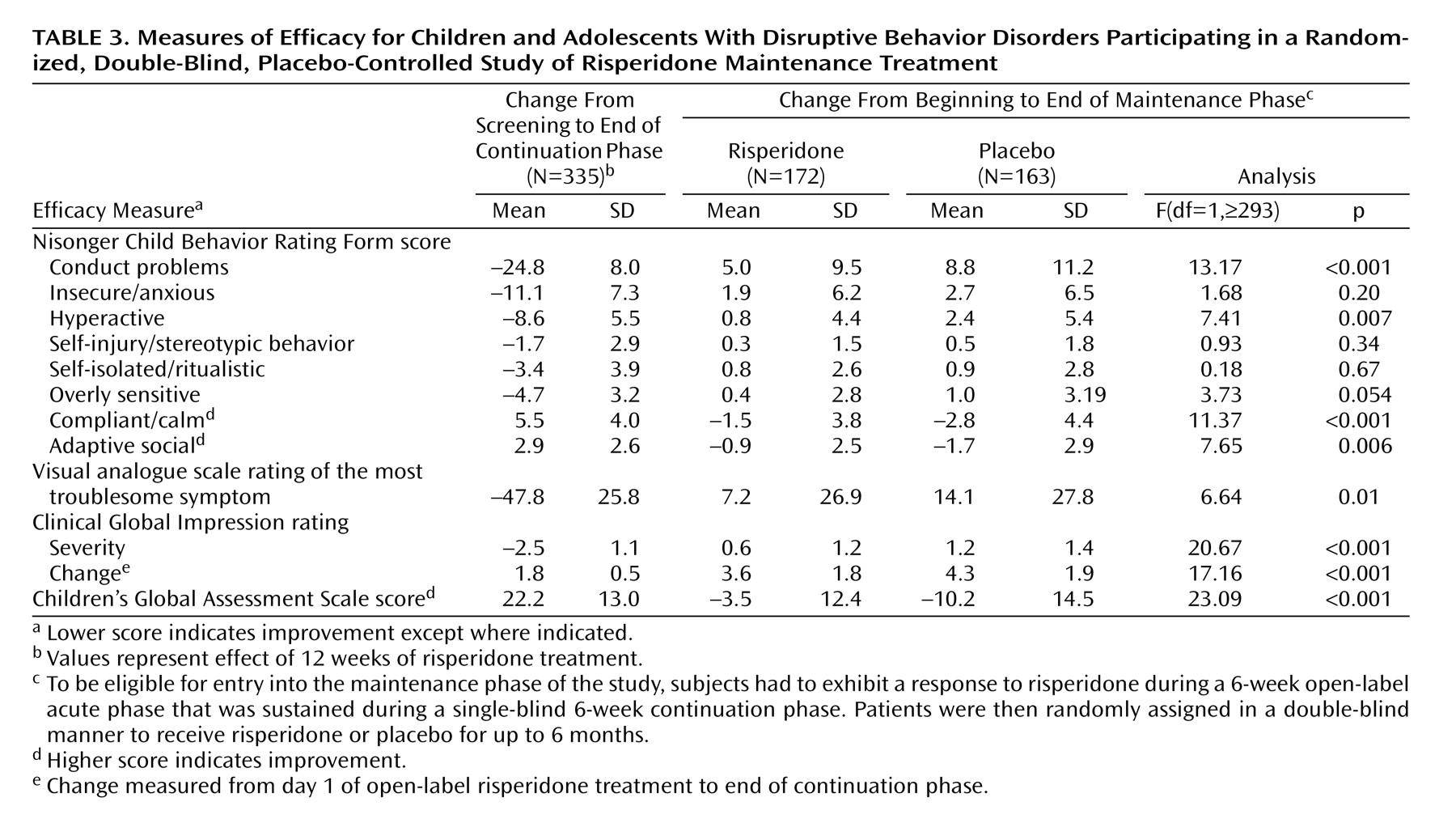
Risperidone was associated with a significantly lower rate of symptom recurrence than placebo (27.3% versus 42.3%) (c
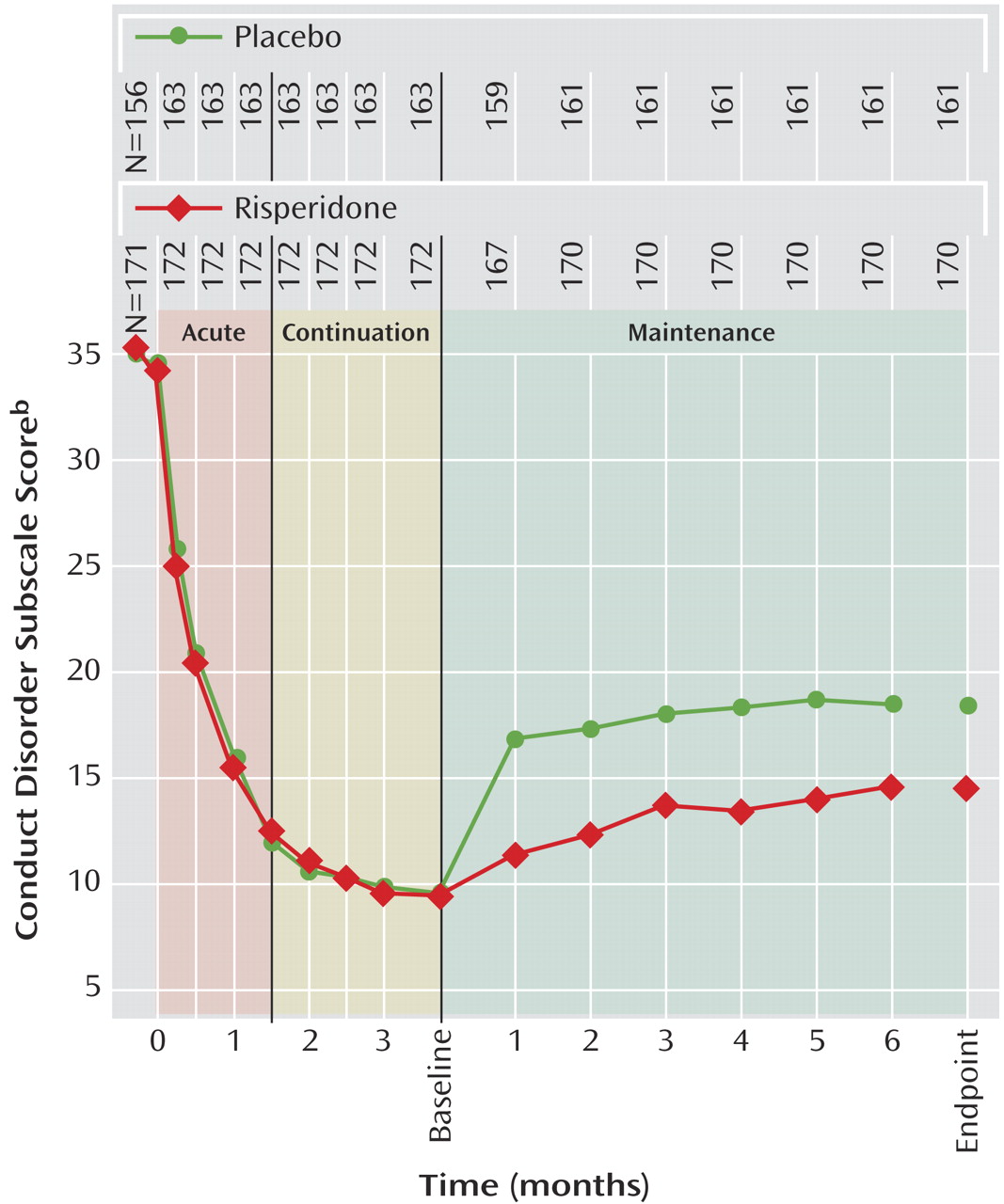
2=10.04, df=1, p=0.002). In the intent-to-treat population, conduct problem scores decreased (i.e., condition improved) significantly within 2 weeks of treatment initiation and continued to improve further throughout acute and continuation treatment (
Figure 3). During the maintenance phase, mean scores increased compared with the start of the maintenance phase in both groups, but deterioration at endpoint of the maintenance phase was significantly higher in the placebo group (F=13.17, df=1,321, p<0.001). Change from the beginning of the maintenance phase to endpoint for all Nisonger Child Behavior Rating Form subscales, the most troublesome symptom visual analogue scale rating, and the global measurements (CGI severity and Children’s Global Assessment Scale) favored risperidone, with significant differences (p≤0.01) between risperidone and placebo on all measures except the insecure/anxious, self-injury/stereotypic behavior, self-isolated/ritualistic, and overly sensitive subscales of the Nisonger Child Behavior Rating Form. The changes in secondary efficacy measures in the intent-to-treat population from screening to the end of the continuation phase and from the beginning of the maintenance phase until endpoint are presented in
Table 3.
Patients maintained on risperidone for up to 9 months had significant improvements (by two decile categories) in mean Children’s Global Assessment Scale scores, corresponding to a change from moderate or severe impairment to generally functioning well.
Safety and Tolerability
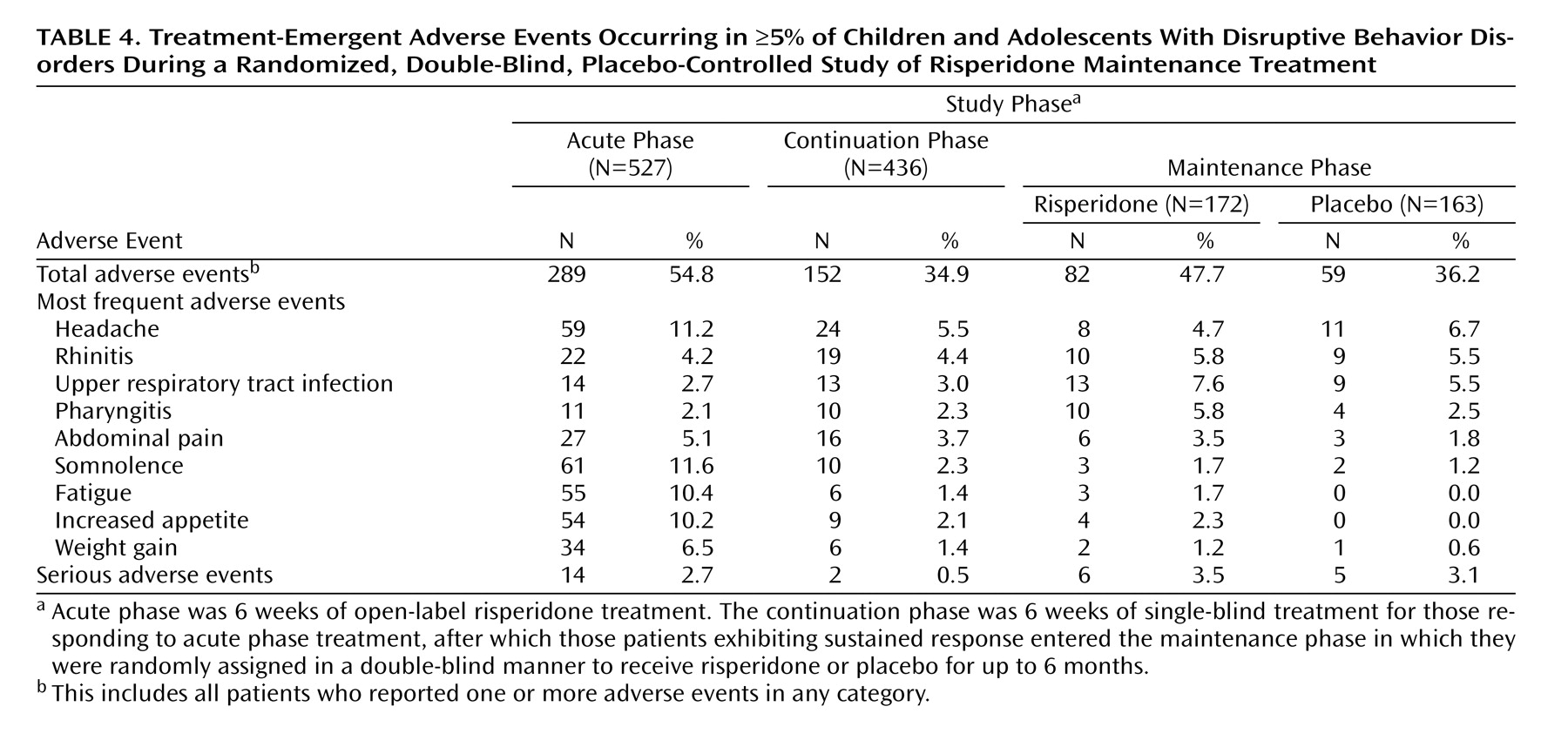
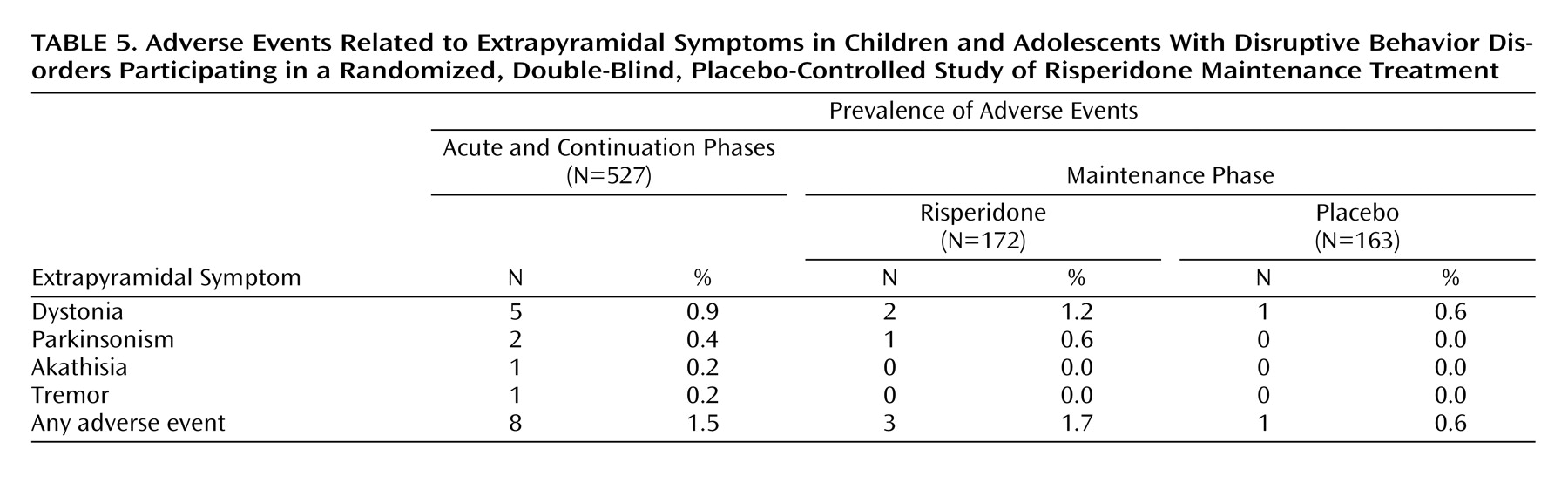
Safety data were available for all 527 patients enrolled in the acute treatment phase. Treatment-emergent adverse events were reported more frequently during acute treatment (54.8%) compared with the continuation phase (34.9%) and maintenance phase (47.7% with risperidone versus 36.2% with placebo) (
Table 4). The most frequently reported treatment-emergent adverse events were headache, somnolence, fatigue, and increased appetite. Most treatment-emergent adverse events were mild or moderate in severity. Treatment-emergent adverse events resulted in treatment discontinuation in 1.7% of patients during acute treatment, 2.1% during the continuation phase, and 1.7% with risperidone and 0.6% with placebo during the maintenance phase. Treatment-emergent adverse events that resulted in treatment discontinuation during the maintenance phase with risperidone were involuntary muscle contractions, abnormal ECG, and paranoid reaction, each of which occurred in one patient. One patient receiving placebo discontinued because of an implantation complication related to treatment for a different condition. The incidence of extrapyramidal symptoms was low (
Table 5), and antiextrapyramidal-symptom medications were used to treat extrapyramidal symptoms in only two patients. There were no reports of tardive dyskinesia. Potentially prolactin-related treatment-emergent adverse events were infrequent and occurred during acute and continuation therapy in 10 patients (six male [gynecomastia] and four female [amenorrhea in one, breast pain and lactation in one, and lactation only in two]) and during risperidone maintenance in three male subjects (gynecomastia) and two female subjects (amenorrhea and lactation in one, and breast discharge in one). There were no potentially prolactin-related treatment-emergent adverse events among those receiving placebo during the maintenance phase. Somnolence was most commonly reported during the acute phase of treatment (11.6%) and dramatically decreased thereafter. In general, somnolence was of mild-to-moderate severity and time-limited to treatment initiation or dose increase.
There was no clinically significant change in mean fasting glucose levels during treatment. Neither were there clinically meaningful changes in other laboratory values with the exception of prolactin plasma levels. Mean prolactin plasma levels increased during acute and continuation phases from 8.3 ng/ml (SD=6.6) at screening to 27.2 ng/ml (SD=20.1) at the end of the continuation phase. In the intent-to-treat population, mean levels decreased during the maintenance phase both for patients treated with risperidone (baseline: 29.4 ng/ml [SD=21.9]; endpoint: 20.3 ng/ml [SD=21.3]) and those receiving placebo (baseline: 29.7 ng/ml [SD=18.0]; endpoint: 9.6 ng/ml [SD=9.5]).
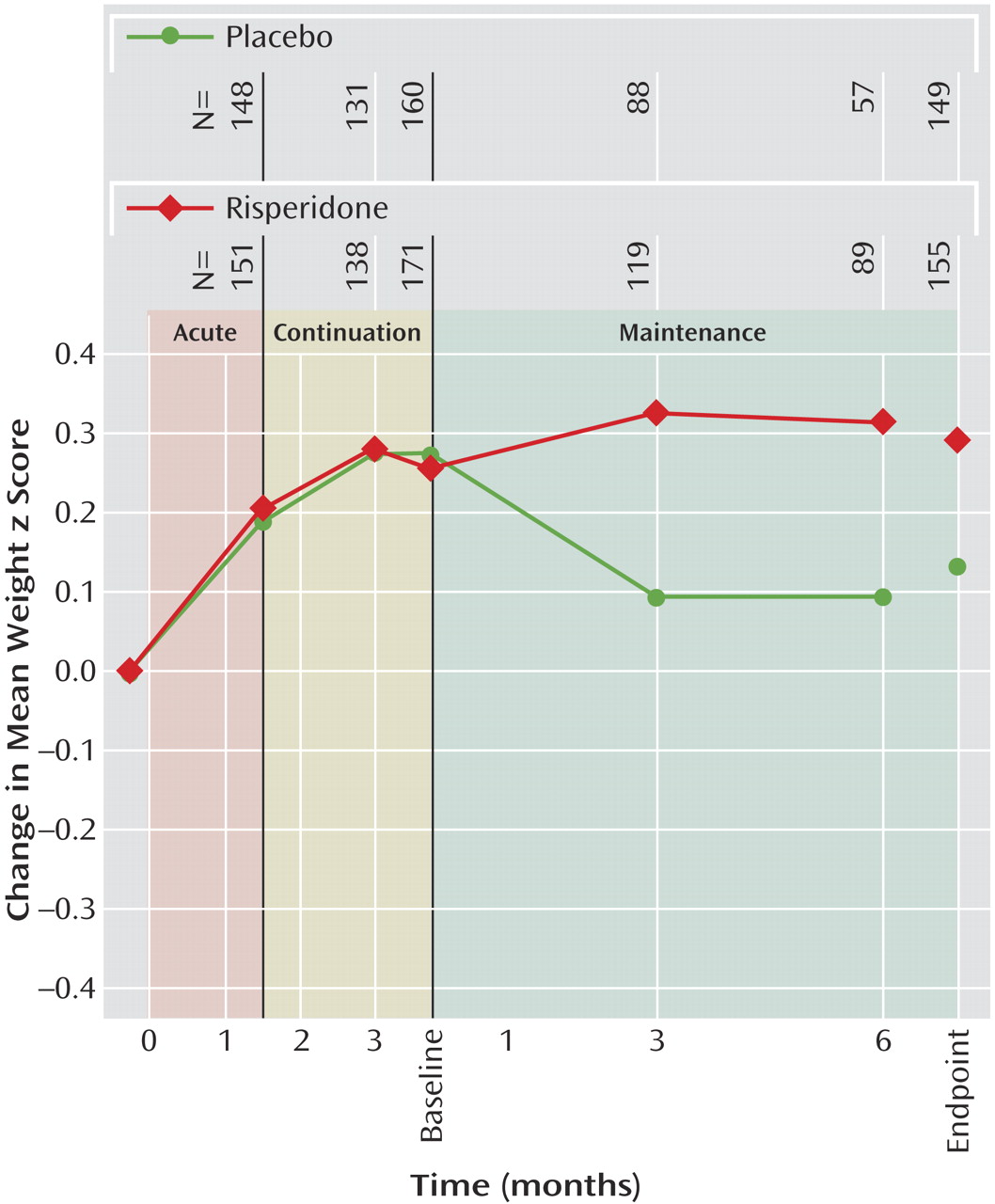
Mean weight increased from screening to the end of the continuation phase (week 12) by 3.2 kg (SD=2.49). Subsequently, from the beginning to the end of the maintenance phase, mean weight increased by 2.1 kg (SD=2.7) for risperidone-treated patients, whereas patients receiving placebo experienced a decrease in mean weight of –0.2 kg (SD=2.2). Mean height and body mass index increased over the first 12 weeks of treatment by 1.7 cm (SD=2.1) and 1.0 kg/m
2 (SD=1.1), respectively. From the beginning to the end of the maintenance phase, height increased in both groups (risperidone: mean=2.5 cm, SD=1.9; placebo: mean=1.5 cm, SD=1.7), and body mass index increased in risperidone-treated patients (0.3 kg/m
2 [SD=1.1]) but decreased for patients switched to placebo (–0.4 kg/m
2 [SD=0.9]). Among patients receiving risperidone in all study phases, mean weight and body mass index z scores increased during the first 12 weeks of treatment (weight: mean change=0.3 [SD=0.3]; body mass index: mean change=0.3 [SD=0.4]). During maintenance treatment no further weight gain beyond natural growth was observed (change in weight z scores from maintenance baseline: mean=0.0, SD=0.3, at endpoint and mean=0.1, SD=0.3, at other time points). In placebo patients, mean weight z scores decreased compared with maintenance baseline by 0.1 (SD=0.2). Mean body mass index z scores showed a similar pattern, with no increase in risperidone patients (mean change from baseline to endpoint=0.0, SD=0.4) and a decrease in placebo patients (mean change=–0.2, SD=0.3).
Figure 4 indicates that weight increase was an initial effect, was not continued with prolonged treatment, and was reversed with discontinuation of risperidone treatment.
There were no clinically significant changes in vital signs and no significant changes in QT intervals during any treatment phases. Most mean changes in ECG parameters were small, and the incidences of high heart rate (>100 bpm) and low PR interval (≤120 msec) during the double-blind maintenance phase were similar between the risperidone and placebo groups.
Changes in cognitive testing scores from beginning to end of the double-blind maintenance phase were small and similar for the risperidone and placebo groups. Mean changes on the MVLT-15 (total correct scores) were 0.1 (SD=1.7) for placebo (N=139) and –0.3 (SD=3.8) for risperidone (N=138); on the MVLT-10 these were 4.3 (SD=4.9) for placebo (N=5) and 2.0 (SD=2.1) for risperidone (N=9). The scores on the continuous performance test were also similar between placebo- and risperidone-treated patients (mean change in number of correct hits: placebo, mean=0.1 [SD=0.4], N=136; risperidone, mean=0.1 [SD=0.4], N=139). These differences were not statistically significant.
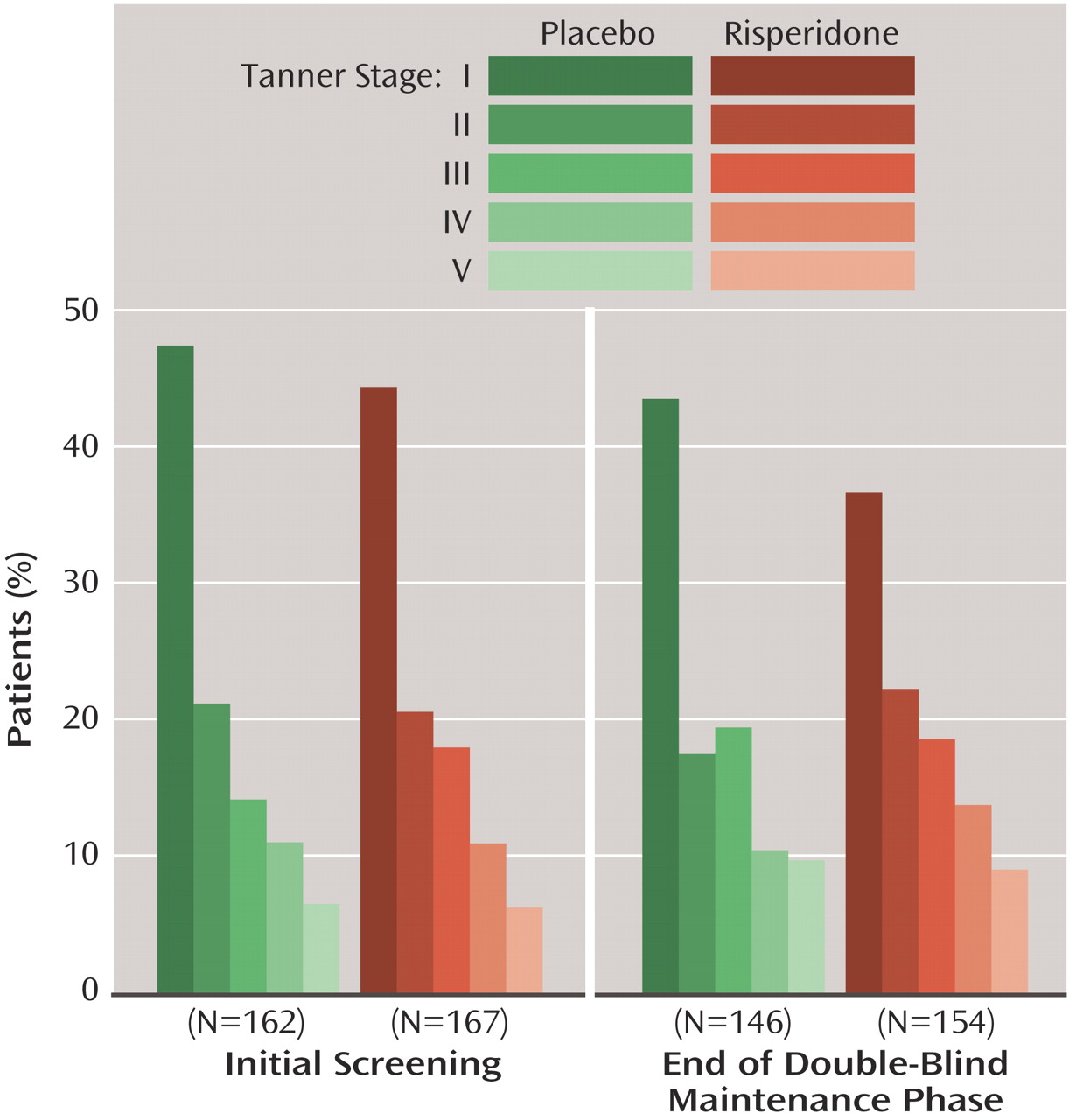
As expected, Tanner stages increased during the study, with similar changes in patients receiving risperidone and placebo during the maintenance phase (
Figure 5).