Appreciating the distinctions between research and usual care is critical for informed consent for research, and its absence has been denoted a “therapeutic misconception”
(1). Research differs from routine clinical care in that the former strives to further knowledge about diseases, whereas the latter focuses on meeting individual patients’ needs. Participants manifesting therapeutic misconception may fail to attend to important “disadvantages to participating in clinical research that stem from the nature of the research process itself”
(1), namely, that the study design may compromise patients’ personal interests (e.g., by random assignment rather than by individualized treatment assignment) for the sake of scientific integrity
(2). Despite disagreement regarding whether—and under what conditions—therapeutic misconception should invalidate consent
(3), participants’ ability to distinguish between research and usual clinical care seems essential to the consent process. Failure to make these distinctions may result in underestimation of risks or overestimation of benefits of research participation, hindering informed decision making.
Therapeutic misconception appears to be widespread
(3–
10). In a study specifically designed to identify therapeutic misconception, Lidz and colleagues
(7) interviewed 225 subjects enrolled in 44 varied, mostly nonpsychiatric, clinical protocols (including patients with depression but not schizophrenia). The investigators reported that 31% of the participants expressed inaccurate beliefs regarding the degree of individualization of their treatment, whereas 51% manifested an unreasonable belief in the nature or likelihood of the benefits given the methods of the study in which they were enrolled. A total of 62% of the participants were judged to manifest therapeutic misconception on one (N=93) or both (N=46) of these bases
(11). Older age, lower education, and worse self-described health were risk factors for therapeutic misconception. Neuropsychological correlates were not specifically examined in this study, so it was unclear how cognitive factors related to therapeutic misconception.
Given expanding numbers of clinical trials involving people with schizophrenia and other mental illnesses
(12), it is important to know whether potential subjects may be misperceiving key points about participation and whether there may be areas of particular difficulty for individuals with schizophrenia. It also would be useful to have a measure for screening potential research participants for misconceptions and to ascertain therapeutic misconception’s relationship to other domains of decisional capacity for research.
In this study of older patients with schizophrenia or schizoaffective disorder, we reported on the frequency and degree of participants’ failures to recognize limitations on the individualization of treatment that are inherent in much clinical research
(11). We assessed the prevalence and correlates of inaccurate beliefs regarding the degree of individualization of their treatment (therapeutic misconception) among patients in reference to a hypothetical placebo-controlled, double-blind study of an experimental antipsychotic medication. We hypothesized that patients with less education, more severe psychopathology, more cognitive impairment, or worse performance on measures of decision-making capacity would show a higher prevalence and degree of therapeutic misconception.
Method
Subjects
The participants were 87 patients recruited and consecutively enrolled as part of a larger consent-enhancement study of middle-age and older patients with schizophrenia and schizoaffective disorder. Inclusion criteria were ages ≥50 years and a DSM-IV diagnosis of schizophrenia or schizoaffective disorder. Exclusion criteria were a lack of fluency in English or the presence of dementia. The participants were recruited through board-and-care residences, academic and county psychiatric clinics, and the local veterans affairs hospital. Both inpatients and outpatients were eligible to participate, as were patients under legal conservatorship.
Informed Consent
The protocol was reviewed and approved by the University of California, San Diego, Human Research Protections Program’s Institutional Review Board. All participants gave informed consent to participate in this study. For individuals on conservatorship (N=3), the conservators also provided permission. This consent study clearly met the federal definition of a minimal risk protocol (45 CFR 46.303). Thus, consistent with the “sliding scale” concept of capacity (requiring a lower threshold for consent to minimal risk protocols
[13]), the institutional review board did not require explicit evaluation of capacity for consent to this study. Nonetheless, we used an interactive process to ensure that all participants adequately understood the consent study. A staff member met with potential participants to review the consent form, encourage questions, and clarify any confusion. This was similar to the approach taken by our group and others studying informed consent in patients with mental disorders
(14–
17). (Three potential participants were excluded because they lacked a minimal level of understanding about the nature of this consent study.)
Measures
Therapeutic misconception
The primary dependent measure in the present study was the total score on a six-item scale created by one of the authors (P.S.A.) and his colleagues at the University of Massachusetts Medical School to test specifically for aspects of therapeutic misconception related to a failure to appreciate the limits on individualization of care
(7,
11). The scale was designed to evaluate the presence of therapeutic misconception relative to a protocol that the participant is actually considering. For the present study, we developed a hypothetical clinical trial involving random assignment to one of three study arms. The scale (available from the first author) was administered by a trained member of our research staff who met individually with each participant and read aloud a one-page description of the hypothetical clinical trial (containing all information relevant to providing correct answers to the therapeutic misconception scale items). The staff person stopped to clarify any questions (most involved specific vocabulary terms), and the participant had a copy of the consent form to review. When needed, the staff person made substantial efforts (e.g., encouraging the participant to read along and to ask questions) to ensure that the participant was engaged in the procedure. Because the scale questions were embedded in a lengthier procedure with questions incorporated after designated points in the consent form, the participant was asked several questions assessing his or her understanding of the hypothetical study before the therapeutic misconception scale was administered. We thus attempted to minimize the effects of memory on scale performance, simultaneously giving the participants an opportunity to answer related (but not identical) short-answer questions regarding the hypothetical study.
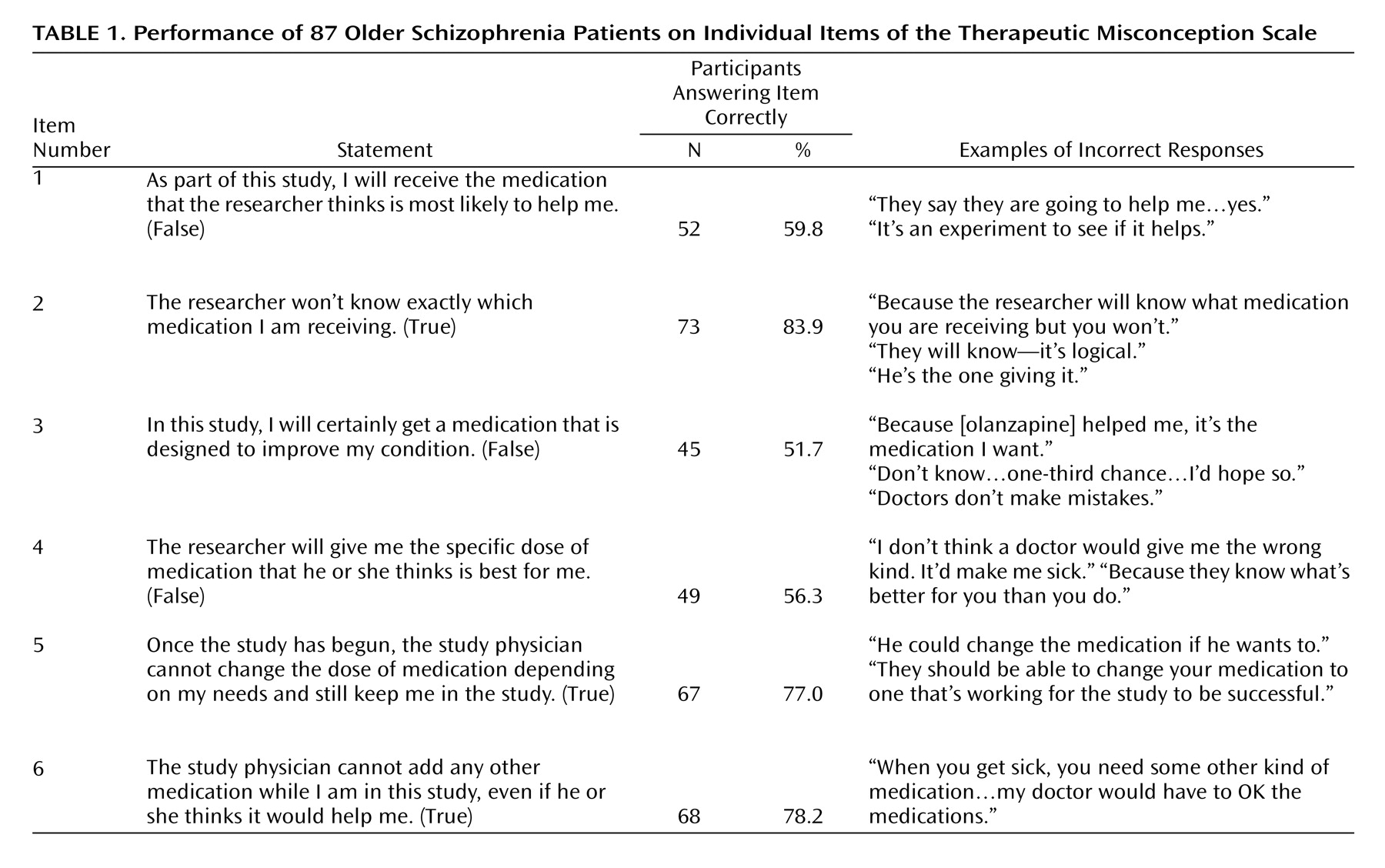
Immediately after disclosure of the consent information, the participants were asked to answer six true/false statements related to therapeutic misconception. We scored each item as “0” (correct) or “1” (incorrect) and then summed the scores. Thus, the possible range was 0 to 6, with higher scores indicating worse performance (i.e., a higher degree of therapeutic misconception). In addition, because we wished to explore whether subjects endorsing
any degree of therapeutic misconception were different in any way from those with
no therapeutic misconception, we separately categorized participants into those two groups. For a subset of 21 individuals, we also asked follow-up questions after each item, thereby gathering narrative data to evaluate the reasoning behind the subjects’ responses (
Table 1).
Decisional capacity
The subjects were administered the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR)
(18) to assess four domains of decisional capacity: understanding (comprehension of information regarding the study), appreciation (grasping the significance of the information for one’s own situation), reasoning (rational manipulation of information by comparing risks and benefits of participating versus not participating and providing likely consequences of one’s choice), and expression of a choice about participation
(13,
19). Each of the MacCAT-CR subscales was considered separately in the following analyses.
Psychiatric symptoms
Severity of psychiatric symptoms was assessed with the Positive and Negative Syndrome Scale
(20) and the Hamilton Depression Rating Scale
(21). The Birchwood Insight Questionnaire
(22) was used to evaluate the participants’ awareness of being mentally ill, the need for treatment, and the idea that “unusual” experiences are symptoms of one’s illness. Higher scores on the Positive and Negative Syndrome Scale and the Hamilton depression scale represent worse symptoms, whereas higher scores on the Birchwood Insight Questionnaire indicate better levels of insight.
Cognitive functioning
Severity of cognitive deficits was evaluated with the Mattis Dementia Rating Scale
(23). The Mattis Dementia Rating Scale provides a total score (range=0–144) and five subscale scores: attention (range=0–37), initiation/perseveration (range=0–37), construction (range=0–6), conceptualization (range=0–39), and memory (range=0–25), with lower scores indicating worse cognitive functioning.
Procedures
All measures were administered by trained research staff. The MacCAT-CR and therapeutic misconception scale were administered by the same rater, but the person administering the psychiatric rating scales and the Mattis Dementia Rating Scale was unaware of the MacCAT-CR and the therapeutic misconception scores. Interrater reliability for the MacCAT-CR and the clinical rating scales is conducted every 6 months as part of routine practice in our research center. For the MacCAT-CR, we examined interrater reliability with a subset of 15 interviews rated separately by three raters. Intraclass correlation coefficients (ICC) for the three major subscales were the following: understanding, ICC=0.98; appreciation, ICC=0.84; and ICC=reasoning=0.78. (There was too little variability in expression of a choice in this group to provide meaningful reliability scores.)
Data Analysis
The patients with schizophrenia (N=54) and schizoaffective disorder (N=33) were combined for the main analyses. (Data from our research group and others indicate that these diagnostic groups share more similarities than differences in neurocognitive functioning [
24–
26]. Also, these diagnoses are often combined in clinical trials, so together they constituted the relevant group for studying consent for schizophrenia research.) We also compared correlates of therapeutic misconception in the two groups.
To assess internal consistency, we calculated Cronbach’s alpha for the therapeutic misconception scale. We evaluated all variables for significant skew and transformed the following variables: the Birchwood Insight Questionnaire score, the Mattis Dementia Rating Scale total and all subscale scores, and the MacCAT-CR subscale score. We used Pearson’s correlations to analyze the associations between performance on the therapeutic misconception scale and demographic, neuropsychological, and psychopathological variables and between performance on the therapeutic misconception scale and performance on the MacCAT-CR subscales. We used analyses of variance (ANOVAs) to examine differences among categorical groups (gender, diagnosis, ethnicity, and marital status) in degree of therapeutic misconception. We used chi-square tests and one-way ANOVAs to assess differences in clinical, decision-making, and neuropsychological factors between subjects without any therapeutic misconception and those with any therapeutic misconception. To minimize the risk of type I error, given the number of correlations and comparisons examined, we chose a relatively conservative alpha level of p<0.01 (two-tailed) to define significance.
Results
Participant Characteristics
The patients’ basic demographic and clinical characteristics are described in the table accompanying the online version of this article. At the time of testing, most (89%) were clinically stable outpatients, although the majority were living in board-and-care facilities, with currently mild negative, positive, and depressive symptoms (the Positive and Negative Syndrome Scale and the Hamilton depression scale) as well as mild to moderate deficits in cognitive function (the Mattis Dementia Rating Scale) and decision-making capacity (MacCAT-CR).
Performance on the Therapeutic Misconception Scale
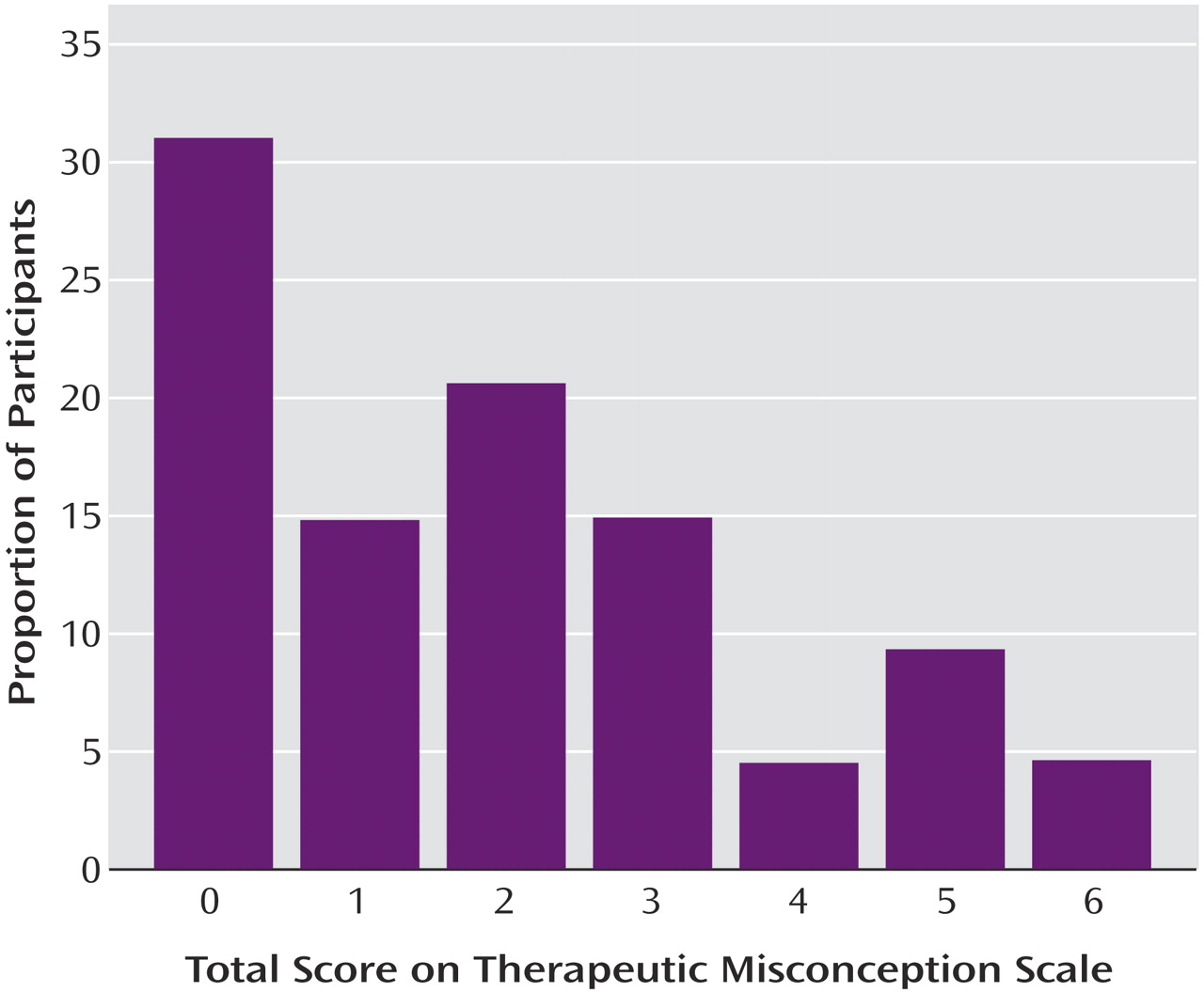
The mean total score on the scale that measured therapeutic misconception was 1.93 (SD=1.82) (possible range=0–6). Twenty-seven (31.0%) of the subjects were categorized as “therapeutic misconception absent”; the remaining 60 (69.0%) were classified as “therapeutic misconception present” because they missed at least one item (
Figure 1). A clear majority of the participants understood that the researcher would be blind to medication assignment (question 2) and that the researcher would be unable to change their medication dose or add another medication (questions 5 and 6) (
Table 1). Just over half of the participants correctly stated that the researcher would not be able to choose the most helpful medication or dose for them personally (questions 1, 4, and 5). Of note, these three items were also the ones requiring an answer of “false.” Internal consistency for the therapeutic misconception scale was fair (Cronbach’s alpha=0.75).
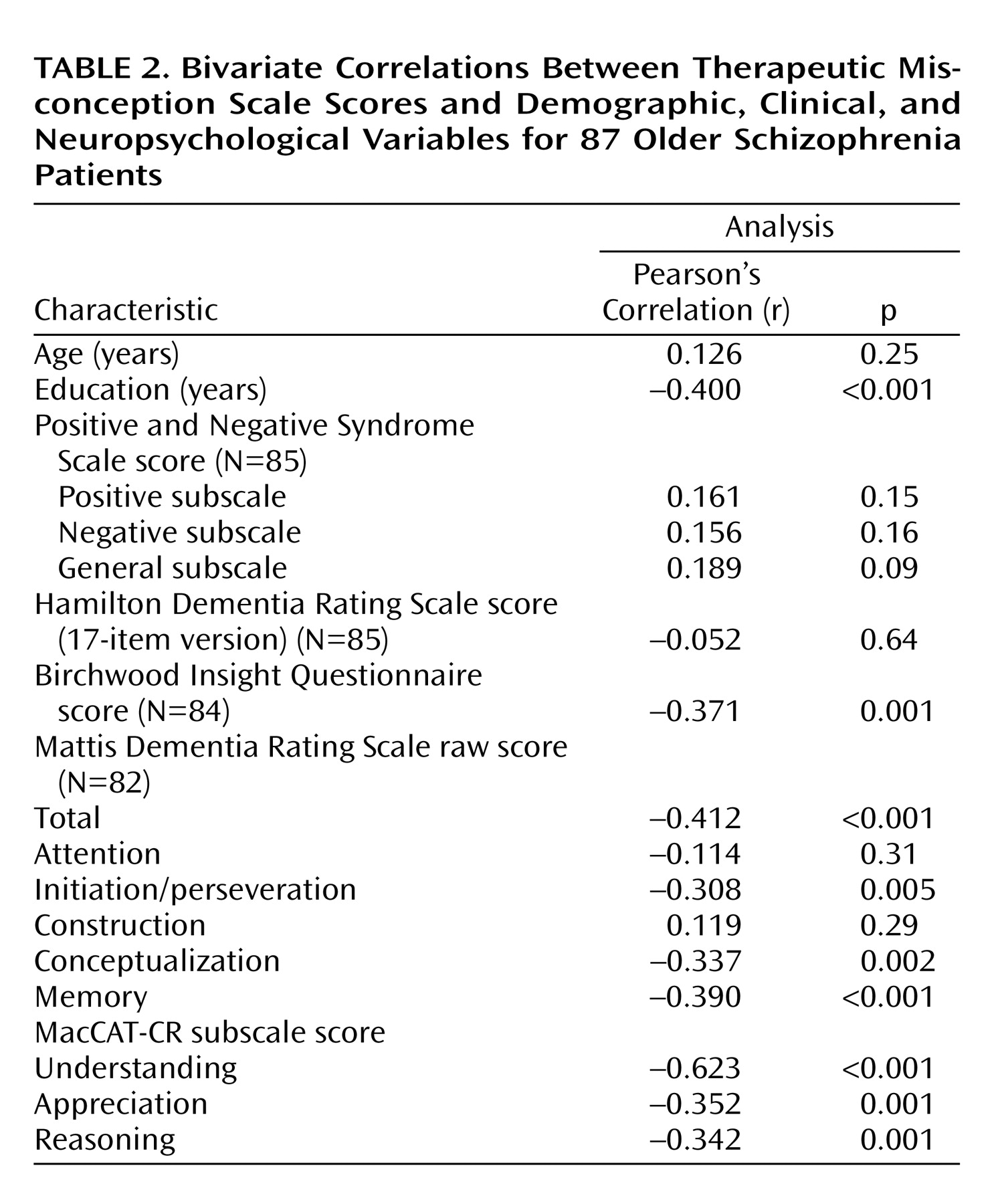
Patient Characteristics and Therapeutic Misconception
The strongest correlates of therapeutic misconception were lower education, worse insight (Birchwood Insight Questionnaire score), overall severity of cognitive deficits (Mattis Dementia Rating Scale total score), and worse decisional capacity (MacCAT-CR understanding, appreciation, and reasoning scores) (
Table 2). (We also found the same pattern of results with nonparametric statistics [Spearman’s correlation].)
ANOVA group comparisons indicated that patients with schizoaffective disorder had less therapeutic misconception than those with schizophrenia (F=6.06, df=85, p<0.02); patients living in an apartment or house also performed better than patients living in residential facilities (F=11.47, df=84, p=0.001). Therapeutic misconception scores did not differ by age, gender, ethnicity, severity of symptoms, inpatient/outpatient status, or marital status. When we examined the diagnostic groups separately, we found that correlates of therapeutic misconception showed the same pattern in the schizoaffective patients as in the group as a whole. Among the patients with schizophrenia, the only differences from the overall pattern were that MacCAT-CR appreciation and reasoning scores were not significantly associated with therapeutic misconception, although Positive and Negative Syndrome Scale positive (r=0.280, p<0.05) and general (r=0.379, p=0.005) subscale scores were correlated with therapeutic misconception scores.
Discussion
We found variable performance on a brief measure to assess the presence and degree of one key aspect of therapeutic misconception (failure to appreciate protocol-related constraints on individualization of treatment, also known as therapeutic misconception) among a group of middle-age to elderly patients with schizophrenia or schizoaffective disorder. Therapeutic misconception scores were correlated with understanding, appreciation, and reasoning subscale scores on the MacCAT-CR, consistent with the notion that the measure assesses areas relevant to capacity to consent to research and suggests convergent validity
(27).
It is important to note that nearly one-third of the participants answered all items correctly, and two-thirds answered four or more items correctly. These results suggest that many people with schizophrenia can do well on ethically relevant assessments in research. Similarly, Roberts and colleagues
(28) found that patients with schizophrenia could distinguish among hypothetical protocols of varying risk-benefit ratios, showing less willingness to enroll in protocols seen as riskier. Thus, the appropriate question is not whether persons with serious mental illness have therapeutic misconception or other consent-related difficulties but rather which patients are likely to have such deficits. In the present study, worse performance on our scale that assessed therapeutic misconception correlated with cognitive deficits, but associations between therapeutic misconception and severity of psychopathology were equivocal (emerging only for the patients with schizophrenia when analyzed separately). This pattern—stronger impact of cognitive versus psychopathological factors on decisional abilities—is consistent with other studies of consent capacity among psychiatric patients
(16,
17,
29). The negative correlation of education with degree of therapeutic misconception in the present study is also consistent with previous findings in other groups
(5,
11,
30).
Insight deficits strongly correlated with therapeutic misconception in this group. Few prior therapeutic misconception or decisional capacity studies have incorporated measures of illness insight. Our finding of a negative association is consistent with the notion that therapeutic misconception is an aspect of the appreciation domain of decisional capacity and that appreciation in turn is conceptually linked to the clinical notion of illness insight
(19,
31). Having the same interviewer administering both the therapeutic misconception scale and the MacCAT-CR may be a potential limitation in the present study. Rater bias may have increased the apparent association between therapeutic misconception and MacCAT-CR scores, although the “true/false” format of the therapeutic misconception scale probably mitigated such effects. Another potential interpretative limitation is that incorrect responses on the therapeutic misconception scale appeared more likely on the “false” items. The wording of these items may have confused some participants, as suggested by the negative association of therapeutic misconception scores with education and cognitive performance. This pattern may also have indicated an affirmative response set, which might have affected performance disproportionately on “false” items. In an informed consent survey, Joffe and colleagues
(4) had a similar concern, but even without such items, they found that items related to therapeutic misconception were more frequently missed than other items. Furthermore, although wording likely affected performance for some participants, this would not explain all of the variance in performance. For instance, among the subset of participants who answered open-ended questions, we found substantive understanding of the underlying concepts in those answering correctly, as well as more definitive evidence of therapeutic misconception in people answering incorrectly. In short, therapeutic misconception in this group did not appear fully attributable to error in measurement.
Another possible limitation was our use of a hypothetical protocol. When they are informed about a hypothetical study, some subjects may pay less attention to details, perhaps partially explaining the substantial prevalence of therapeutic misconception among our group. Also, when participants consider an actual protocol, additional information relevant to understanding that protocol may be communicated during recruitment
(32). Hypothetical protocols, however, are widely used in capacity research
(16,
33,
34) because they confer advantages: prototypic elements can be incorporated, and a wider array of participants (not limited by the inclusion/exclusion criteria of a specific clinical trial) can be enrolled. Our hypothetical protocol was designed to closely resemble actual schizophrenia clinical trials. Moreover, from the narrative data from a subset of participants, as well as from the strong correlation with performance on the MacCAT-CR, it appears that the subjects who understood the protocol did so despite its hypothetical nature.
Our group also was likely less ill (based on Positive and Negative Syndrome Scale scores) than the patients who typically enroll in schizophrenia-related clinical trials
(35,
36). Given the number of clinical trials (especially industry-sponsored trials) enrolling more severely or acutely ill individuals, it would be important to assess therapeutic misconception in a more heterogeneous group.
Despite these limitations, our findings are important in showing the substantial prevalence (73%) of at least some aspects of therapeutic misconception. Together with other researchers’ work, these findings highlight the need for more concerted efforts to ask potential participants about possible misconceptions, misunderstandings, or overestimation of benefits. This will go partway toward fulfilling an obligation to improve methods of unearthing and countering misconceptions in psychiatric (and other) research populations
(7).
Suggestions for countering therapeutic misconception have focused on being more explicit about procedures unique to research (e.g., random assignment or placebos)
(3), particularly because many people have little understanding of these terms
(10,
37). Our findings indicate that an initial target should be the fundamental distinction between research and clinical care because many participants do not appreciate that researchers do not necessarily prescribe treatment based on individuals’ personal needs. Simply defining random assignment and placebos may be insufficient; more detail should be provided about
why these are used, particularly in the study being considered. It has also been suggested that highlighting that payments or other compensation is often given for participation in research may help reduce therapeutic misconception
(38,
39), although empirical evidence is lacking.
Educational interventions for schizophrenia research consent have shown that enhanced procedures—ranging from computer-based presentations incorporating a more structured format with review of key information
(40,
41) to corrected feedback
(42) to a combination of educational approaches
(16)—are effective and lead to patients performing no differently than healthy comparison subjects.
Our results thus echo a prominent theme in the psychiatric research ethics literature
(43,
44): although
potentially more vulnerable mentally ill research participants are not
necessarily impaired, assumptions based on diagnosis alone are unwarranted. This appears relevant to the assessment of therapeutic misconception: identifying and mitigating therapeutic misconception in all research participants should thus be an overriding goal of investigators, regardless of specialty or participant population.