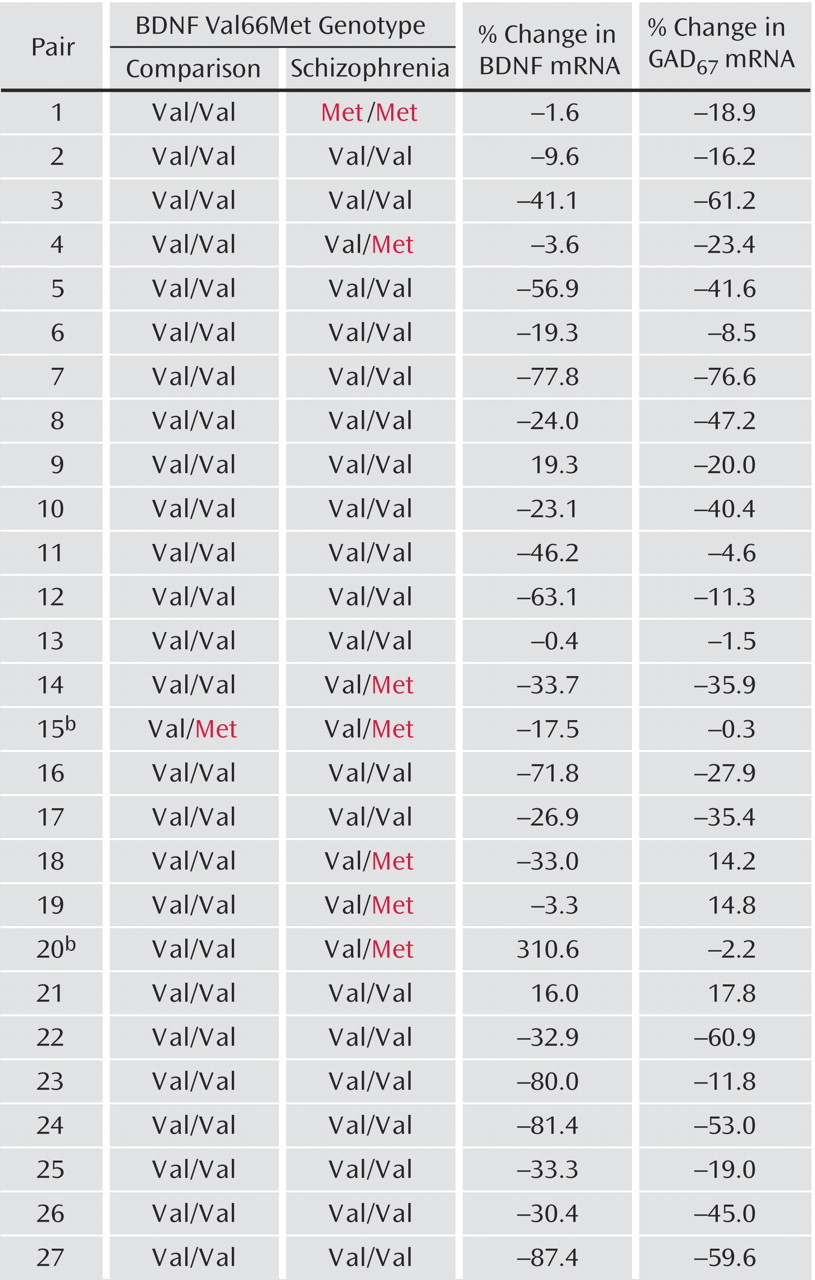
Although the primary focus of this study was to assess the effect of
bdnf genotype on GAD
67 mRNA expression, we unexpectedly encountered a significantly lower frequency of the
bdnf Met66 allele in the comparison group (2%) than in the schizophrenia group (15%). However, previous case/control studies using larger populations reported much higher frequencies (19%–22%) of the Met66 allele in comparison subjects that were similar to
(9) or even higher than
(11) the frequency found in schizophrenia subjects. Considering the limited size of our cohort, it seems likely that the low frequency of the Met66 allele in the comparison group represents a chance finding.
Because BDNF has been shown to induce GAD
67 expression in cortical neurons in vitro and in vivo
(5,
6), the decreased expression of GAD
67 mRNA in the prefrontal cortex of subjects with schizophrenia might reflect a reduced availability of BDNF. BDNF signaling in the cortex is determined not only by its synthesis in neurons but also by its release from them. Thus, GAD
67 expression levels might be modified by the BDNF Val66Met genotype, given that the Val to Met substitution in the 5′ prodomain of pro-BDNF peptide reportedly causes abnormal intracellular trafficking and a subsequent reduction of activity-dependent secretion of BDNF
(9). Thus, we expected that the magnitude of decrease in GAD
67 mRNA would be greater in schizophrenia subjects with the Met allele after the level of BDNF mRNA expression was controlled. However, no evidence for such an effect was found, suggesting that the possible deficit in activity-dependent release of BDNF caused by the presence of the Met allele does not contribute to the decrease in GAD
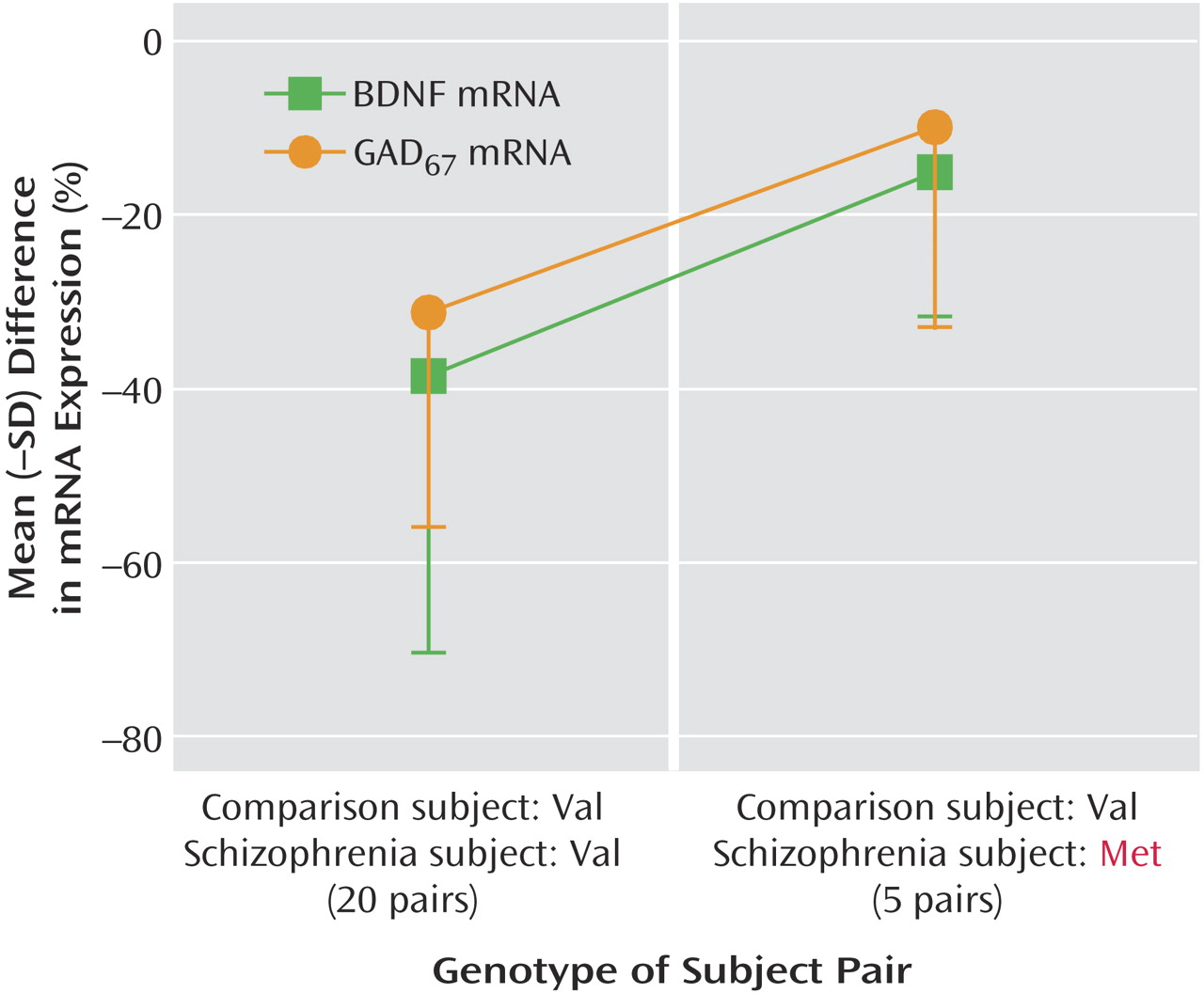
67 mRNA expression in subjects with schizophrenia. It is interesting that the decreases in both GAD
67 and BDNF mRNA expression in the prefrontal cortex appear to be less severe in schizophrenia subjects with the Met66 allele than in those without the Met66 allele. Further studies are needed to both confirm this observation and to illuminate potential mechanisms underlying the association between the presence of the Met allele and a tempering of the deficit in GAD
67 and BDNF mRNA expression in subjects with schizophrenia. These findings, together with our previous observation of no change in cortical GAD
67 mRNA expression in mice with a neuron-specific inducible knockout of
bdnf (8), suggest that the reduced availability of BDNF may not be a primary cause of decreased GAD
67 expression in the prefrontal cortex of subjects with schizophrenia. In contrast, other factors such as the availability of TrkB
(8) or allelic variants in the GAD
67 gene
(12) may be more important.