Borderline personality disorder is a devastating condition, affecting 1%–2% of the population and causing tremendous disruption of patients’ lives and relationships
(1) . Emotional and behavioral dyscontrol play a large role in the morbidity and mortality of this condition. Affective dysregulation in individuals with borderline personality disorder manifests itself as emotional instability with a propensity toward intense negative emotional states (anger, anxiety, and dysphoria)
(2) . Borderline patients also demonstrate a range of impulsive behaviors (self-mutilation, parasuicidal behavior, substance abuse, sexual promiscuity, and binge eating), particularly in the setting of negative affective states. Impulsivity (and/or impulsive aggression) is considered to be an underlying dimension in borderline personality disorder and best predicts the persistence of borderline psychopathology across time
(3) .
Core elements of the psychopathology of borderline personality disorder have been defined and described in a model of serious personality disorders developed by members of our group
(4) . The model posits a dynamic interaction of temperament (individual differences in motor and emotional reactivity and self-regulation), a preponderance of negative affect, low effortful control, and an absence of a coherent sense of self and others
(2) . In language bridging psychological and neurobiological perspectives, Depue and Lenzenweger
(5) have conceptualized borderline personality disorder as an emergent phenotype principally reflective of a complex interaction involving diminished positive emotion in relation to increased negative emotion, in interaction with diminished activity of the modulatory constraint system and exaggerated reactivity of the fear system.
The neural substrates of borderline personality disorder are not well understood but have received greater attention in recent years. A number of (resting) [
18 F]fluorodeoxyglucose positron emission tomography (PET) studies have described decreased dorsolateral prefrontal activity and increased or decreased medial and ventral prefrontal and temporal activity
(6 –
9) . Increased amygdala activation to negative stimuli has also been described
(10,
11), as has limbic response to traumatic memories
(12) . A preliminary study of more extended frontal activation during response inhibition has also been reported
(13), and in a PET drug challenge study with patients with intermittent explosive disorder and borderline personality disorder
(14), orbitofrontal and amygdalar dysfunction were correlated. A recent neuropsychological study of patients with borderline personality disorder, as well as patients with orbitofrontal and non-orbitofrontal prefrontal lesions, suggested that impulsivity and negative affect in borderline personality disorder may be related to orbitofrontal dysfunction
(15) . The functioning of prefrontal control mechanisms in the setting of negative emotional states, which is of particular relevance to borderline personality disorder, has not been probed selectively with functional magnetic resonance imaging (fMRI) techniques.
Emotional responsivity and inhibitory control have been studied with animal models and are amenable to human brain mapping. Although studies of the cognitive control of emotion
(16), as well as many functional neuroimaging studies of emotional processing and behavioral inhibition, have been published recently, relatively few have addressed the interaction of the latter two functions (e.g., references
17 –
19 )—an interaction that plays an important role in the regulation of human behavior in health and disease, particularly in borderline personality disorder.
Method
Participants
Participants were 16 patients with borderline personality disorder (15 of them female; 15 of them right-handed; mean age=31.25 years, range=19–50 years) and 14 healthy comparison subjects (10 female; 12 right-handed; mean age=23.8 years, range=18–31 years). Borderline diagnoses were confirmed with the International Personality Disorder Examination
(21) (criteria score range=5–9, dimensional score range=10–18; mean=14.9, SD=2.28). None of the participants had medical or neurological conditions. Comparison subjects had no psychiatric disease as assessed by the Structured Clinical Interview for DSM-IV and the International Personality Disorder Examination. Other current diagnoses among the borderline patients included panic disorder (N=3), social phobia (N=1), specific phobia (N=1), posttraumatic stress disorder (N=1), obsessive-compulsive disorder (N=1), generalized anxiety disorder (N=1), somatization disorder (N=1), alcohol abuse (N=2), and cannabis abuse (N=1). None of the patients had any current substance dependencies. Past diagnoses included major depressive disorder (N=8), anorexia nervosa (N=4), bulimia (N=2), obsessive-compulsive disorder (N=2), posttraumatic stress disorder (N=2), generalized anxiety disorder (N=1), alcohol dependence (N=2), and sedative/hypnotic dependence (N=1). On the International Personality Disorder Examination, other categorical diagnoses included paranoid (N=7), antisocial (N=2), histrionic (N=5), avoidant (N=6), narcissistic (N=3), dependent (N=3), and obsessive-compulsive (N=2) personality disorders.
There was no significant difference in gender composition between the two groups, but there was a significant difference in age (p=0.012). Age was therefore incorporated, along with gender and handedness, as a covariate in the imaging data analysis. Eleven patients were taking psychotropic medications (eight were taking antidepressants, four mood stabilizers, two antipsychotics, and one an anxiolytic). Four patients and one comparison subject were using oral contraceptives. Additional analyses were performed with covariates included for the most prevalent medications (antidepressants and mood stabilizers), past axis I history (major depression and anorexia), and other International Personality Disorder Examination categorical diagnoses (paranoid, histrionic, and avoidant personality disorders). The comparison subjects in this study were the same individuals whose findings we reported in an earlier article focusing on the interaction of inhibition and emotion in healthy subjects
(20) .
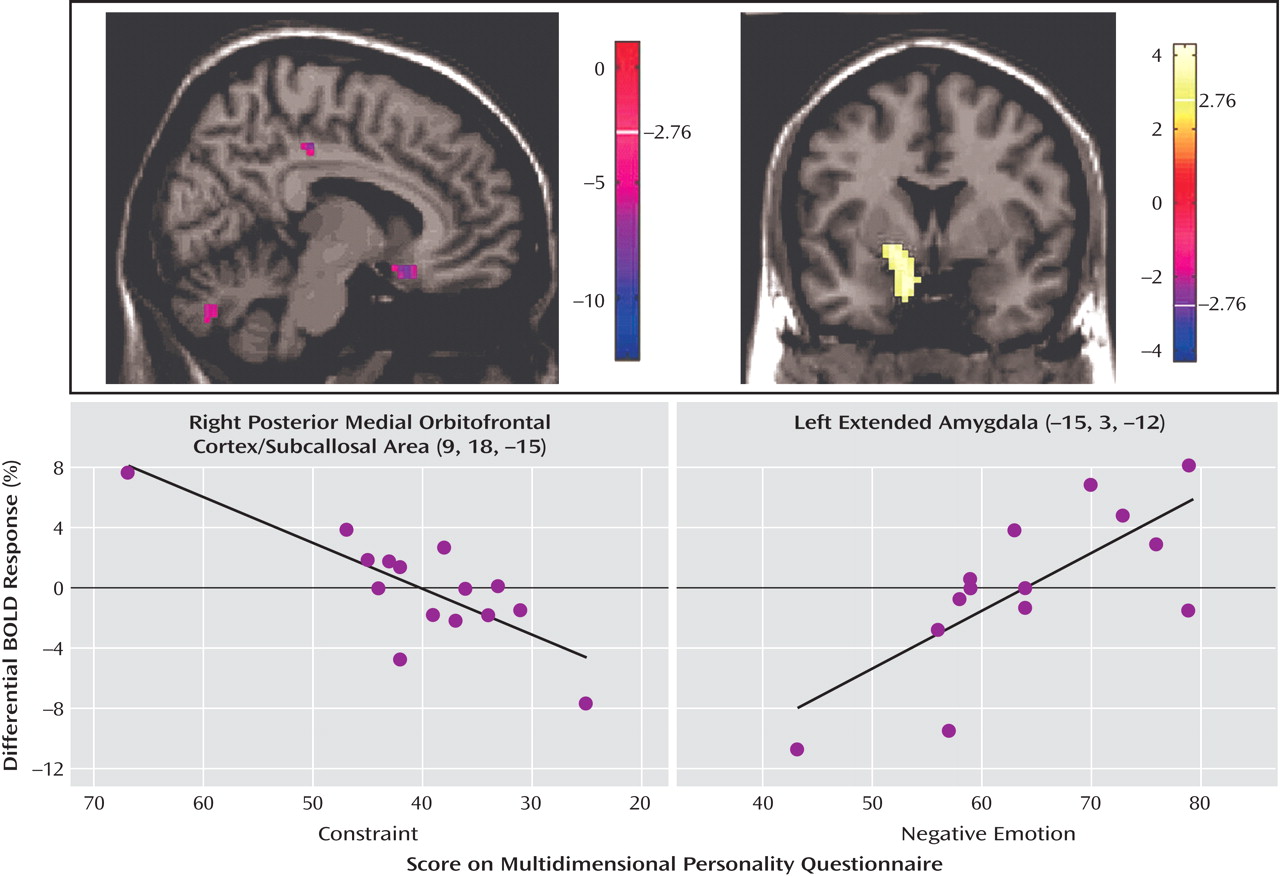
The Multidimensional Personality Questionnaire was used to relate specific clinical symptom measures to functional neuroimaging results with a focus on negative emotion and on constraint. Negative emotion is a construct that taps proneness to experience anxiety, anger, and related states of negative engagement. Constraint is a construct reflective of control and harm avoidance; a high level of constraint reflects tendencies to inhibit and restrain impulse expression. All participants gave informed consent before enrollment in the study, which was part of a protocol approved by the institutional review board of New York Presbyterian Hospital and Weill Medical College of Cornell University.
fMRI Paradigm
Participants underwent scanning while they performed an emotional linguistic go/no-go task developed to investigate neurocircuitry underlying the interaction between emotion and motor inhibition
(19,
20), with verbal stimuli containing themes salient for individuals with borderline personality disorder. Behavioral response was based on orthographically based cues: participants were instructed to perform a right-index-finger button-press immediately after (silently) reading a word appearing in normal font (go trial) and to inhibit this response after reading a word in italicized font (no-go trial). Button-press responses and reaction times were recorded. A total of 192 distinct linguistic stimuli were used (64 negative, 64 positive, 64 neutral). Words were balanced across all valence conditions for frequency, word length, part of speech, and imageability.
The task was presented in a block design comprising 24 blocks (six blocks per run, four runs total). The six blocks per run represented the six main conditions (neutral go, neutral no-go, negative go, negative no-go, positive go, positive no-go), the presentation of which was counterbalanced to control for order and time effects across runs. Go blocks contained 16 go trials (100% go trials), and no-go blocks contained 10 go trials (62.5% go trials) and six no-go trials (37.5% no-go trials), presented in pseudorandomized order to establish prepotent motor response yet have ample no-go trials. Each word was presented individually in white letters on a dark background for 1.5 sec followed by a 0.75-sec interstimulus interval (total block duration=36 sec). Each block was followed by a 20-sec rest period during which a fixation cross was displayed. A shortened practice run using different words preceded the experimental runs to ensure that participants understood and could follow the task instructions.
When the scanning was completed, participants were removed from the scanner and instructed to perform a word recognition task. They were given a list of the 192 stimulus words (targets) randomly interspersed with 48 distractor words (divided equally into negative, positive, and neutral categories, balanced for the same linguistic qualities as targets) and asked to circle the words they believed they saw during the scanning session. They were then given a word valence rating task, which was also made up of both target and distractor words, and asked to rate the valence of each word on a 7-point Likert-like scale (–3=very negative, 0=neutral, +3=very positive).
Image Acquisition
Imaging data were acquired with a GE Signa 3-T MRI scanner (General Electric Company, Waukesha, Wisc.; maximum gradient strength 40 mT/m, maximum gradient slew rate 150 T/m per sec) at the Weill Medical College of Cornell University. Structural images were acquired with a three-dimensional high-resolution T 1 -weighted spoiled gradient-recalled acquisition sequence (resolution 0.9375×0.93751.5 mm 3 ). Echo planar imaging (EPI) was used to obtain blood-oxygen-level-dependent (BOLD) functional MR images. After shimming to maximize homogeneity, a series of gradient echo fMRI scans was acquired (repetition time=1200 msec, echo time=30 msec, flip angle=70°, field of view=240 mm, 15 slices, slice thickness=5 mm, interslice distance=1 mm, matrix=64×64), with a z-shimming algorithm to reduce susceptibility artifact at the base of the brain (modified from reference 22). A reference T 1 -weighted anatomical image with the same slice placement and thickness and a matrix of 256×256 was acquired immediately before the EPI acquisition.
Image Processing and Data Analysis
Prior to data analysis, customized statistical parametric mapping software (London, Wellcome Department of Imaging Neuroscience;
23 ) was used to realign functional EPI scans based on intracranial voxels, coregister functional images to the corresponding high-resolution anatomical image based on the transformation of the reference anatomical image to the latter for each individual subject, perform stereotactic normalization to Montreal Neurological Institute (MNI) space based on the high-resolution anatomical image, and spatially smooth with an isotropic Gaussian kernel (full width at half maximum=7.5 mm).
A two-stage general linear model was used to examine the effect sizes of the key group/condition contrasts. First, a voxelwise multiple linear regression model was used at the individual subject level. This model included the principal regressors of interest, which consisted of the stimulus onset times convolved with a prototypical hemodynamic response function, and the covariates of no interest, which consisted of the temporal first-order derivative of the principal regressors, global fluctuations, realignment parameters, and scanning periods. The temporal global fluctuation estimated as the mean intensity within brain of each volume was removed through proportional scaling. Temporal filtering was performed to counter the effects of baseline shifts and higher-frequency noise, and a first-order autoregressive (AR[1]) model of the time course was used to accommodate temporal correlation in residuals. Effects at every brain voxel were estimated by a least squares algorithm, and the effect images for each condition were then combined in a series of linear contrasts to be entered into the second-stage, group-level analysis. Second, at the group level, a random-effects model was used, which accounts for intersubject variability and allows population-based inferences to be drawn. The within- and between-group effects of the hypothesis-driven contrasts were estimated using a least squares algorithm with demographic variables (age, gender, and handedness) incorporated as covariates in the context of an analysis of covariance. These group-level effect estimates generated t-statistic maps, and their statistical significance was evaluated based on random field theory. The statistical inferences were thresholded at a voxelwise p value and cluster extent (p<0.005, uncorrected for multiple comparisons, and a cluster extent of four voxels with a voxel volume of 27 mm
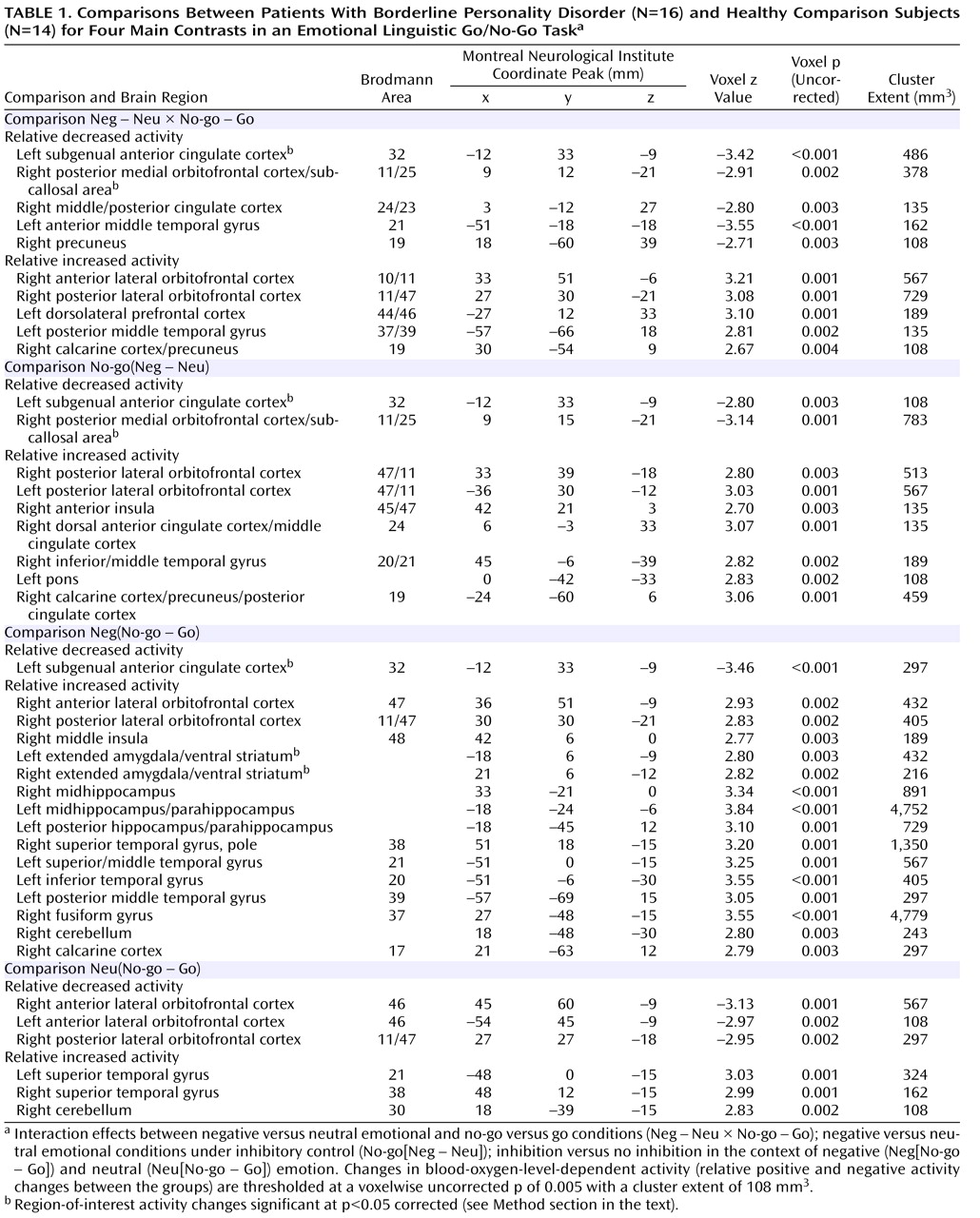
3 ). Based on a priori hypotheses, regions of interest were the amygdala, the (subgenual) anterior cingulate gyrus, and the medial orbitofrontal cortex; these were defined on the basis of previously reported functional imaging studies concerned with impulse control and negative affect regulation
(24,
25) . Regions of interest were examined by correcting the voxelwise p value at the local maximum of the nearest cluster
(26) . Behavioral data—response times, error rates, recognition rates, and valence ratings—were analyzed using repeated-measures analysis of variance and subsequent Wilcoxon signed rank-sum tests to focus on marked performance differences across groups and conditions.
Discussion
This study was specifically designed to probe the interaction between behavioral inhibition and negative emotion in patients with borderline personality disorder. Based on core clinical features of the disorder as well as behavioral neuroscientific and psychological models, we hypothesized that patients would show a deficit particularly in the function of the ventromedial prefrontal cortex for this clinically salient interaction, which we previously demonstrated to be active in healthy subjects
(20) .
The behavioral results verify the participants’ attention to, and effortful performance of, the tasks and that the no-go condition achieved inhibitory tone (as reflected in reaction times). Borderline patients rated the negative emotional words (tailored to borderline psychology), but not the positive or neutral words, more negatively than healthy comparison subjects. This finding is consistent with a previous psychological study
(27) and supports the validity of the probe. Although overall performance did not differ significantly between patients and comparison subjects, under no-go block conditions, reaction times were slightly longer for patients. While all participants performed the task well, the patients had more errors of omission (for neutral and negative no-go) and commission (for negative no-go) than the comparison subjects. These findings suggest that patients had greater difficulty with the behavioral task demands.
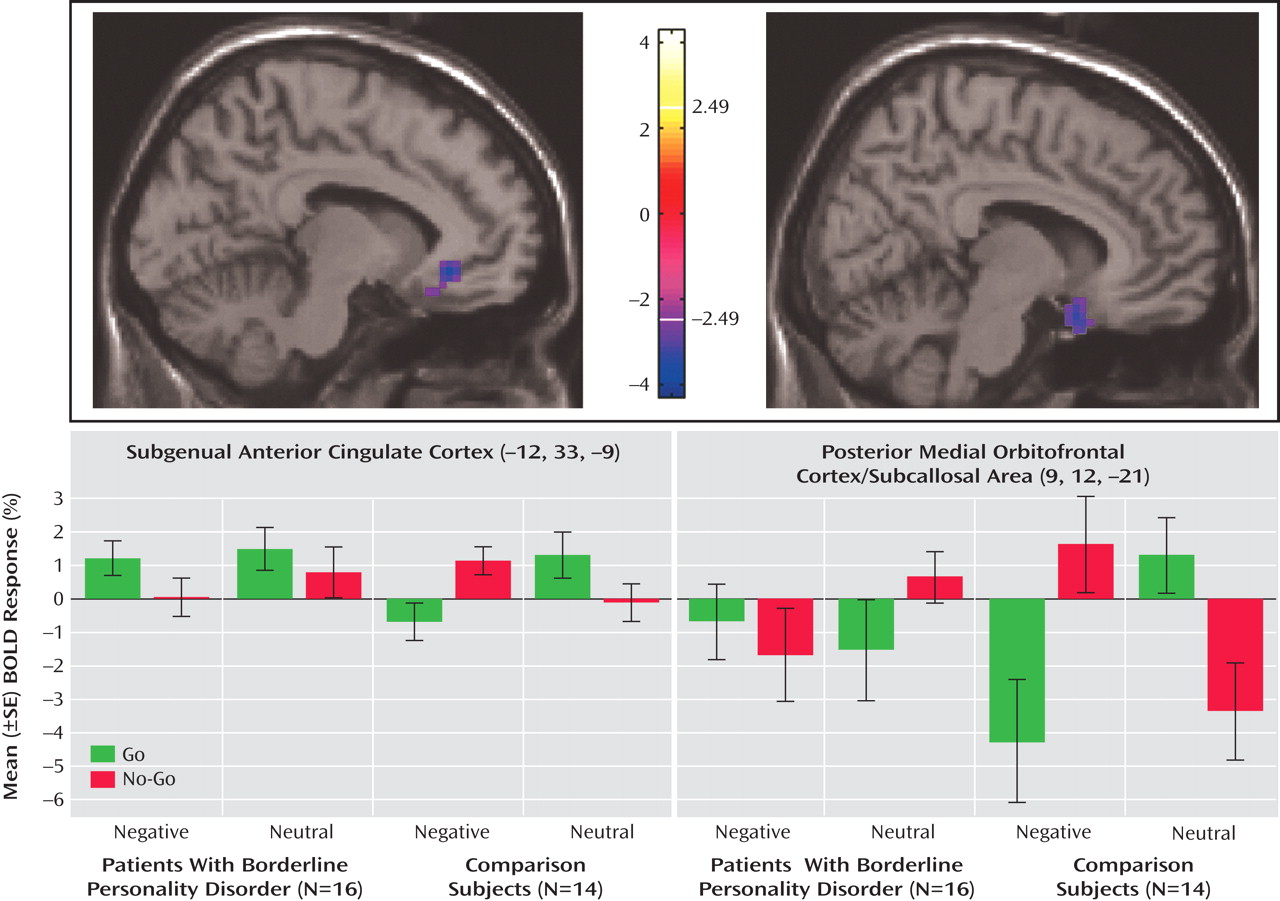
The neuroimaging results demonstrate a deficit (compared with healthy subjects) of activation in the medial orbitofrontal cortex associated with inhibitory task demands in a negative emotional context in the borderline patients. Furthermore, decreasing activity was highly correlated with the Multidimensional Personality Questionnaire measure of decreased constraint in patients. While activity in the medial orbitofrontal cortex was decreased in borderline patients compared with healthy subjects in terms of the behavioral inhibition/negative emotion interaction effects described above, activity in the lateral orbitofrontal cortex was increased.
A medial/lateral distinction emerges from anatomical connectivity of the orbitofrontal cortex, with the medial orbitofrontal cortex subserving behavioral responses in the context of viscerosomatic function and the lateral region mediating sensory-evaluative function
(28) . Projections from the basolateral amygdala (where sensory information converges with affective memory) to the orbitofrontal cortex and then to the central amygdala (which modulates hypothalamic function) and connections between the orbitofrontal cortex and the hypothalamus form pathways by which the orbitofrontal cortex can modulate primitive approach/avoidance behavior as well as higher-order behavior. We previously noted an inverse relationship between medial and lateral orbitofrontal cortex activation under these experimental conditions
(19,
20) . Given the above connectivity distinctions, the medial/lateral profile observed in borderline patients may be associated with their increased responsivity to environmental stimuli. Such an imbalance between the contribution and control of internal states and external experiences may contribute to the emotional and behavioral volatility of borderline patients. This can be seen in the context of a rich clinical literature associating orbitofrontal cortex lesions or dysfunction with socioemotional dyscontrol, reflecting impaired integration of context-relevant emotional information in response-selection processing
(29) .
The subgenual anterior cingulate cortex, just superior to the medial orbitofrontal cortex (including subcallosal area), has received increasing attention for its role in emotional modulation and its dysfunction (and change with treatment) in major depression
(30,
31) . We recently noted a sexual dimorphism in the functioning of this region under negative emotional conditions
(32), which may be relevant given the increased incidence of borderline personality disorder, like depression and anxiety disorders, in women. This region, also highly interconnected with the amygdala
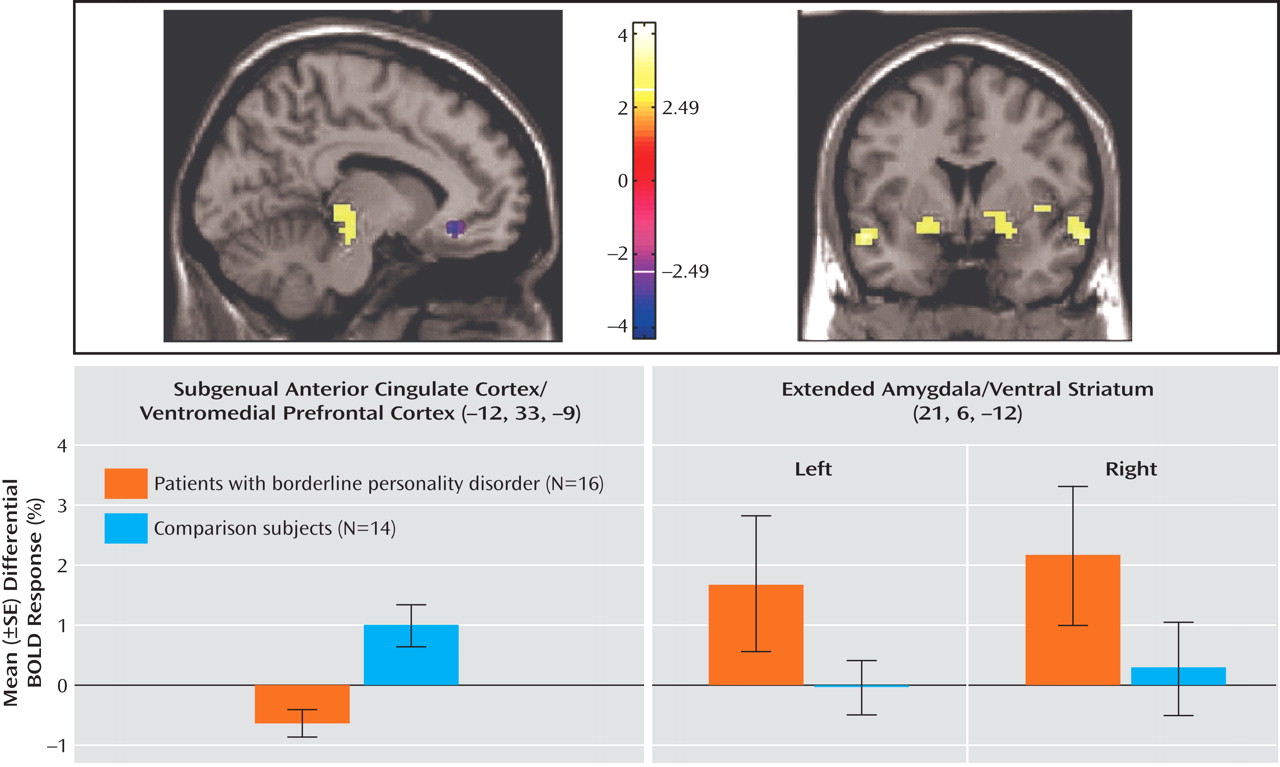
(28), is thought to be the homologue of the ventromedial frontal region in rodents, in which lesioning results in increased fear conditioning and decreased extinction. The failure of normal activation in this region may therefore also be relevant for the breakdown in emotional behavioral control in borderline personality disorder. Ventromedial prefrontal cortex dysfunction in borderline patients, specifically within the medial orbitofrontal cortex and subgenual anterior cingulate cortex, may provide a common (or potentially unifying) locale for both emotional and behavioral dyscontrol.
Conversely, relative amygdalar hyperactivity, comparable to previous studies
(10,
11), is seen here. Importantly, the amygdalar findings in this study were part of a broader area of increased activity in closely related regions ranging from the ventral amygdala through the extended amygdala to the ventral striatum. The close anatomical and functional relationships among these highly interconnected regions underlie the crucial transition and integration from emotion and salience to motivation and behavior
(33) . The ventral (corticobasolateral) amygdala preferentially reacts to clearly negatively valenced, biologically relevant information and tends to correlate with affective as well as symptom measures, such as in depression
(34,
35) . Consistent with those previous results, negative affect in borderline patients correlated with the right corticobasolateral amygdala (differential contrast [No-go(Neg – Neu)]) in addition to the extended amygdala and the ventral striatum, which suggests a bias of negative valence of relevance processing correlating with the severity of this symptom. Models of human extended amygdala function have been proposed in which this region preferentially responds to environmentally salient but ambiguous stimuli
(36,
37) . Activation is seen here in the group comparison under conditions of behavioral inhibition in the setting of negative emotion (Neg[No-go – Go]). This might reflect pathological assessment of saliency detection guiding approach/avoidance in borderline personality disorder, leading to a dysfunction of behavioral/output (not just the perceptual) components of emotional processing, and suggests another contributing, bottom-up substrate for disordered emotional behavior in borderline personality disorder (with the ventromedial prefrontal cortex finding representing a failure of top-down modulation).
In this context, the ventromedial prefrontal cortex (subgenual anterior cingulate cortex/medial orbitofrontal cortex) and the amygdala can have a reciprocal functional relationship, with the ventromedial prefrontal cortex playing a top-down inhibitory role
(38) . This may be the case in the differential negative no-go versus negative go contrast, where borderline patients showed a profile of decreased activity in the subgenual anterior cingulate cortex and increased activity in the extended amygdala. It is notable that in the differential contrast [No-go(Neg – Neu)], the between-group difference appears to be driven by a failure of borderline patients to show the decrease in amygdalar function seen in the healthy comparison subjects. This may provide a mechanism whereby emotion unduly interferes with behavior and cognition in borderline patients and is analogous to reciprocal suppression models of cognitive/emotional processing discussed for other disorders, such as depression and anxiety disorders
(30,
31,
39,
40) .
In borderline patients, the dorsal anterior cingulate cortex showed greater activity in the presence of negative emotional stimuli, particularly in the setting of inhibitory demands, than in comparison subjects. This finding is consistent with a model of domain-specific mapping of prefrontal function, with more dorsal regions involved in more cognitive, conscious, effortfully controlled tasks and more ventral regions involved in more social-emotional, unconscious control tasks
(41,
42) . The negative emotional state may place greater competing demands on response selection processes in borderline personality disorder patients, and their automatic control mechanisms may be dysfunctional.
A limitation of this study is that 11 of the 16 borderline patients were taking medications that were necessary for clinical reasons. Condition-specific activity in the hypothesized regions correlated significantly with the severity of target symptoms, which were present despite medications, and a random-effects model was used, which, while more statistically stringent, addresses a number of factors associated with intersubject variability and makes results more generalizable. Nevertheless, additional analyses were performed with the most prevalent medications as covariates of no interest to address this potential confound. As noted above, the main between-group frontolimbic findings remained significant.
The difference in age between the two groups is also a limitation. Participants were neither in the adolescent nor the geriatric range, and age was incorporated as a covariate of no interest in the analysis to address potential age-related variance, although this may not eliminate all potential between-group age effects. It may be relevant to note that clinical features such as impulsivity tend to diminish with age in borderline patients
(43), which suggests that younger borderline patients might show even more of the condition-specific abnormalities. Comorbid diagnoses, as seen in most borderline patients
(1) and reflecting an overlap of clinical (and probably biological) features, represent another issue to consider. Core neuropsychiatric pathophysiological features were demonstrated despite this variance. As noted above, additional analyses that included the most prevalent additional diagnoses did not significantly alter the main between-group results. Nevertheless, it will be important in the future to conduct studies with additional patients, to extend and test the replicability of these findings, and to further address the age, medication, and comorbidity issues.
In conclusion, the findings of this study provide plausible systems-level neural mechanisms underlying a core clinical difficulty that borderline patients have concerning behavioral dyscontrol in negative affect states. Such hypothesis-driven study of borderline personality disorder with specifically tailored fMRI probes can help elucidate the systems-level pathophysiology of this devastating disorder. It can also help provide a foundation for more targeted diagnostic and treatment strategies.