How This Case Fits Into the Literature
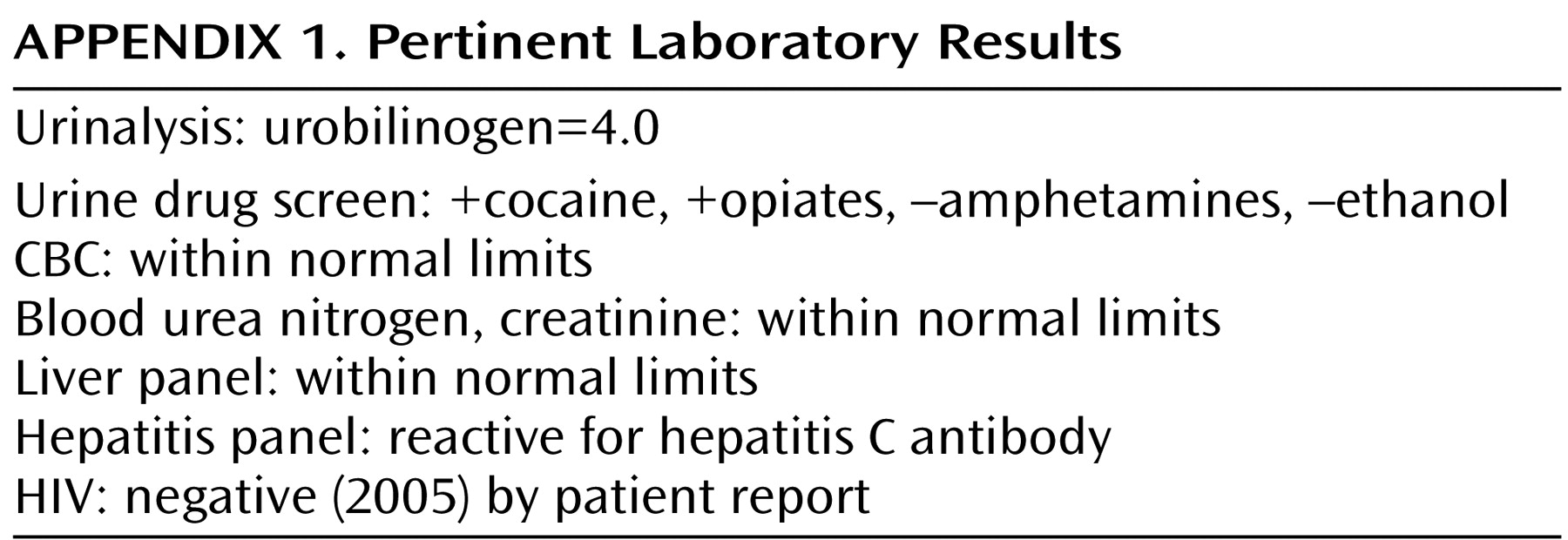
In M.C.’s case, as in many delusions of parasitosis cases, confident categorization of his symptoms is challenging given his inconsistently self-reported psychiatric history (none, schizophrenia, and depression) and positive drug screens. In particular, case reports have linked cocaine psychosis with delusions of parasitosis (distinct from classic formication)
(15) ; delusions of parasitosis have also been reported in the context of schizophrenia
(3,
16) . Taken together, suspicion is high for a secondary organic and/or functional etiology. The potential interaction between multiple secondary factors in delusions of parasitosis is unknown. A secondary etiology would help explain M.C.’s younger age (the mean is the sixth decade of life), earlier presentation in disease course (the mean is 1 year), and unusual claim of ocular involvement
(7,
17) . Although inadequate follow-up complicates interpretation, M.C.’s apparent co-occurring somatic delusions of weight loss and urine odor represent interesting phenomena; one other report exists of delusions of parasitosis with the delusion of body odor in a schizophrenic patient
(16) . Phenomenologically, though, M.C.’s insistence of a dermatologic condition with detailed delusions, his skepticism of psychiatric involvement, and the persistence of the condition comport with current reports.
A controversial phenomenon possibly related to delusions of parasitosis inspiring discussion and media attention is Morgellons’s disease. As in delusions of parasitosis, patients describe insects/parasites crawling on or under the skin, are convinced they are infested and contagious, and produce physical “evidence” of infestation. In particular, though, patients complain of fibers extruding from the skin; such particles produced for examination have been variously identified as cellulose, fibers with “autofluorescence,” fuzz balls, specks, granules,
Strongyloides stercoralis, Cryptococcus neoformans, “alternative cellular energy pigments,” and various bacteria. In no case, however, has an infectious etiology for these mysterious symptoms been confirmed. Morgellons’s disease is largely regarded in the dermatology literature as a manifestation of delusions of parasitosis (and potentially a means of promoting patient rapport through destigmatization), despite the efforts of the Morgellons Research Foundation to promulgate an infectious rather than a neuropsychiatric etiology. Until a treatable infectious component is identified, patients can continue to be treated with neuroleptics—pimozide, risperidone, aripiprazole—which have been reportedly effective
(18,
19) .
Current Etiologic Hypotheses
The debate over the classification and etiology of delusions of parasitosis has revolved around a central question: do delusions of parasitosis reflect a delusional system that arises in response to anomalous sensory perceptions (sensorial view), or does a primary delusion “induce” supporting hallucinations (cognitive view)
(4,
8) ? Etiologic hypotheses have arisen addressing this paradox, and it now appears likely that both mechanisms contribute in different patients, suggesting that delusions of parasitosis are not a homogenous entity. For years, the dopamine D
2 receptor has been implicated in the genesis of psychotic symptoms, including the characteristic of delusions of parasitosis. Involvement of the D
2 receptor in the mesolimbic system could account for the successful use of typical and atypical antipsychotics in treating the primary delusional disorder
(6) . This has been traditionally viewed as support for the cognitive view of delusions of parasitosis. However, it is now believed that pimozide, a highly potent typical antipsychotic and the classic treatment for delusions of parasitosis, antagonizes central opiate in addition to D
2 receptors, which may mitigate pruritus and formication that underlie/accompany the delusion of infestation
(6,
20) . In fact, pimozide has effectively treated formication in the absence of delusion (e.g., in delirium tremens). Furthermore, naloxone, an opioid receptor antagonist
(21), has been used to treat cases of delusions of parasitosis, supporting the sensorial view.
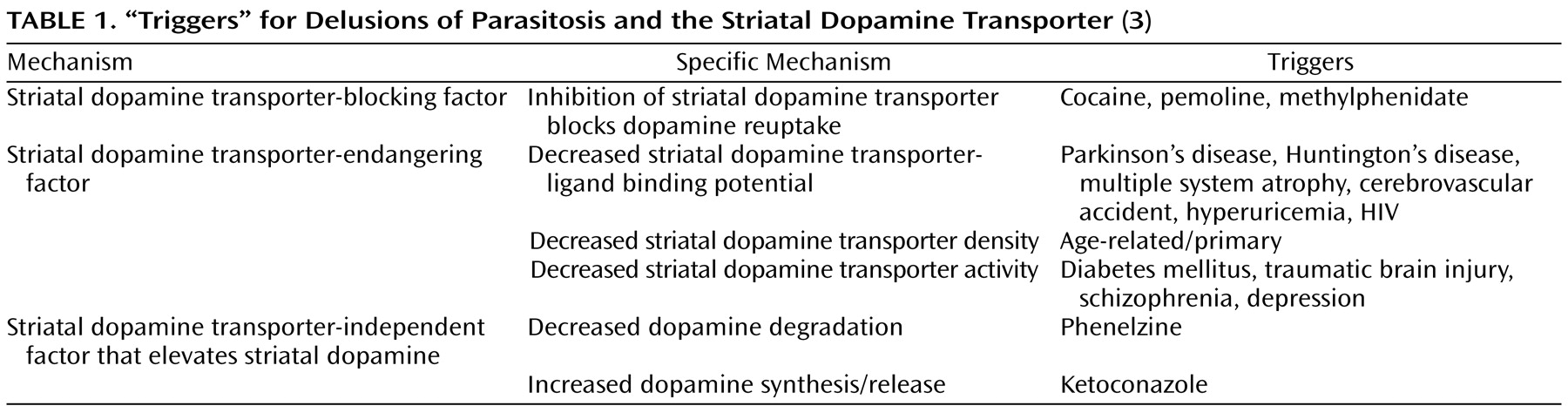
A recently proposed hypothesis focuses on the decreased function of the striatal dopamine transporter. This presynaptic protein is responsible for dopamine reuptake and hence modulates the concentration of synaptic dopamine and the duration of synaptic signals. It follows that decreased functioning of the transporter results in dysregulation of extracellular dopamine concentration. The authors propose that both primary and secondary delusions of parasitosis develop because of dysfunctional striatal dopamine transporter
(3) . Primary forms result from an exaggeration of age-related striatal dopamine transporter density decrease (there is a mean physiologic decrease in striatal dopamine transporter density of 6%–8% per decade), which would help explain the prevalence of cases in patients over 50
(3,
6) . Secondary etiologies of delusions of parasitosis affect the striatal dopamine transporter through a variety of mechanisms (
Table 1 ). Psychostimulants, for example, are known to inhibit the striatal dopamine transporter, resulting in increased dopamine levels. Psychiatric disorders such as schizophrenia and depression are thought to decrease striatal dopamine transporter binding
(3) .
Other studies offer a connection between delusions of parasitosis and cerebral hypoperfusion documented by single photon emission computed tomography. It has been found that active delusional thought is associated with hypoperfusion of the left temporal and parietal lobes. Blood flow normalizes with the resolution of the delusion
(22) . Some argue that successful treatment of delusions of parasitosis with paroxetine and clomipramine, which may restore blood flow to hypoperfused regions by increasing serotonin levels, supports this hypothesis
(22,
23) .
An Integrated, Individualized Model of the Formation of Delusions of Parasitosis
The majority of case reports and reviews of delusions of parasitosis have concentrated on defining a demographic profile, investigating associated conditions, describing response to treatment, and suggesting neurophysiologic etiologies. Given the growing evidence and interest for cognitive and affective models of delusion formation, maintenance, and content
(24), an integrated, neuropsychiatric narrative of M.C.’s delusions of parasitosis is fruitful and contextualizes the particular presentation and course of illness.
First, it is reasonable to regard M.C. as a psychosis-prone individual. Although it remains undocumented, his stated history of schizophrenia is supported by complaints of command auditory hallucinations and prior treatment with antipsychotics. It is not possible to determine with confidence the etiology of M.C.’s past psychiatric history due to presumed long-term drug use. Indeed, it is well-documented that cocaine increases the risk for psychotic experiences (one of Freud’s patients treated with cocaine developed delusions of parasitosis)
(25) . The most common form of cocaine-induced psychosis is paranoid delusions. Studies have identified risk factors for true psychosis formation in cocaine users as male sex, lower body mass index, earlier initiation of regular use, and greater degree of use
(12) .
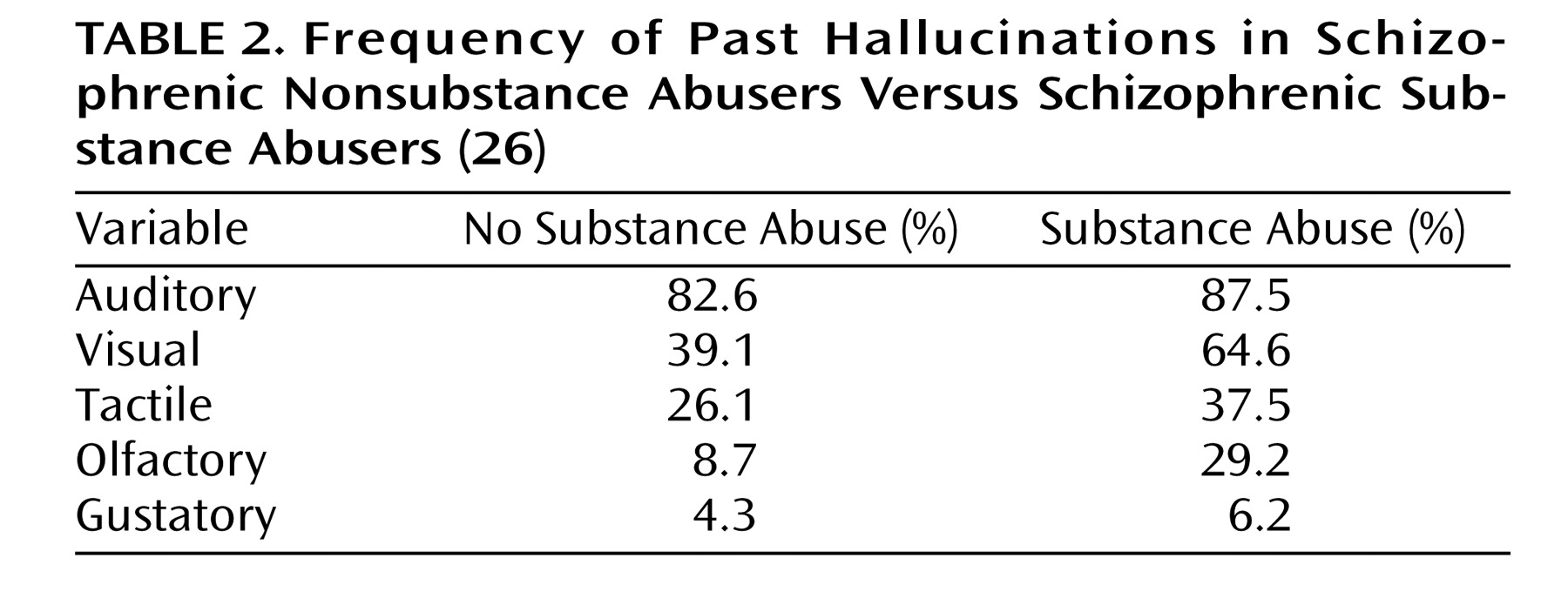
It seems intuitive that M.C.’s psychosis proneness and substance use contributed in concert to the development of his delusions of parasitosis. It has been found that in schizophrenic patients, chronic substance abuse correlates with significantly increased rates of visual and olfactory hallucinations (
Table 2 ). Furthermore, substance abuse is associated with decreased medication responsiveness in schizophrenic and bipolar patients with auditory and tactile hallucinations
(26) . Perhaps this is related to the previously discussed findings that schizophrenia and cocaine act at the striatal dopamine transporter in distinct ways to increase dopamine levels
(3) . In addition, it has been posited that auditory and tactile hallucinations are associated with changes in the superior and inferior temporal lobes in schizophrenia patients; drug use may contribute to the development of psychosis in these compromised individuals by accelerating brain tissue loss
(26) .
How exactly these biologic findings lead to psychosis formation remains unclear. Growing evidence for cognitive dysfunction and bias in delusions has led to a number of cognitive models describing delusion formation. Significantly, cognitive models help explain why delusions, which make sense for the believer and are held to be evidentially true, are often resistant to change despite psychopharmacologic treatment
(24) . Over the last decade, research has unveiled a number of cognitive biases in those prone to psychosis. Patients with auditory hallucinations have been shown to have impaired source monitoring (the ability to discriminate between one’s internal verbal thoughts and other stimuli)
(27) . Source monitoring in psychosis-prone patients has also been shown to be excessively influenced by external factors and suggestion
(28) .
Cognitive theories have also been posed to account for the formation of delusional thoughts in addition to that of hallucinations, and one could easily apply these theories to the formation of delusions
regarding hallucinations. One early study showed that delusional patients showed a “jumping-to-conclusions” reasoning bias, whereby initial probabilistic estimates and subsequent revision of hypotheses were made based on less evidence than in control subjects, a finding that is stronger when reasoning regarding emotional material
(24) . In the formation of paranoid delusions, significant attention has been paid to attributional biases in delusion-prone individuals. Most people are neither rational nor fair minded when generating and selecting causal explanations for events, demonstrating a “self-serving bias” toward internal (self) blame for positive events and external blame for negative events. Paranoid patients have an
exaggerated self-serving bias
(27) . Although incompletely understood, it has been shown that when cognitive resources are taxed or individuals feel threatened, people tend to resort to external attribution. Finally, studies have found that latent inhibition is particularly disrupted in acute psychosis and suggest that an impairment in attentional filtering might underlie symptom development (e.g., M.C. preferentially attended to threatening tactile stimuli)
(24) .
A number of current models approach delusion formation as a variation of
normal belief formation. It has been argued that perceptual disturbance is a necessary “first factor” in delusion formation and that cognitive biases influence the content of hypothesis generation. A necessary “second factor” accounts for how unusual hypotheses result in full-blown delusions (i.e., how does M.C. go from feeling like he has bugs on his skin to a deeply held conviction of infestation?); this factor is thought to be a deficit in the rational evaluation of candidate hypotheses
(24) .
A heuristic model for the acute development of delusions of parasitosis in M.C. can be derived from a synthesis of the two-factor cognitive model of Bell et al.
(24) and some of what is known about the neurobiology of psychosis and substance use. Consistent with such a model would be a situation in which M.C. woke up on the morning of admission experiencing formication related to cocaine use and/or opiate-induced pruritus. He regarded this as an anomalous, threatening experience, and in the context of the stressor of losing his housing, preferentially attended to this aversive stimulus. The disorientation of unfamiliar surroundings as well as his cognitive and attributional strategies that constituted his psychosis proneness biased hypothesis generation toward externalization and concretization. An error in probabilistic reasoning resulted in delusion formation of parasitosis, a hypothesis that comports with M.C.’s persistent denial of cocaine use. Factors that favor maintenance of the delusion include cognitive reinforcement (e.g., the literature recommends against empirically diagnosing or treating delusions of parasitosis patients for scabies)
(9,
29) and continued “anomalous” sensory perception with resumption of drug use. Such a heuristic model generates implications for designing therapeutic intervention. Rigorous attention should be invested in M.C.’s housing troubles, and of course, intensive treatment of his substance use is critical. Furthermore, once the patient’s baseline cognitive strategies removed from substance use become clearer, appropriate attempts at disrupting the maintaining factors of the delusion could be made, including education and long-term antipsychotics as needed.