Acetylcholine acts at muscarinic and nicotinic cholinergic receptors. These receptors are distributed throughout the brain; their locations include the neocortex, hippocampus, thalamus, and basal ganglia
(3), regions that have been implicated in the neural substrate for cognitive processes. The cholinergic system has been implicated in the regulation of attention, memory, processing speed, and sensory gating
(4,
5), processes that are impaired in schizophrenia. Several lines of evidence suggest that the cholinergic system may be disrupted in schizophrenia. Postmortem studies have demonstrated alterations in muscarinic receptor and nicotinic receptor availability or expression
(6 –
9) . In a study using single photon emission computed tomography, the number of muscarinic receptors was significantly lower than normal in the cortex, thalamus, and basal ganglia of people with schizophrenia
(10) . Acute nicotine administration has been shown to improve attention, verbal and visual memory, and working memory in people with schizophrenia
(11,
12) . Furthermore, in a recent study, acute challenge with an α
7 nicotinic receptor partial agonist, DMXB-A, was shown to improve cognitive function and sensory gating
(13) . In combination, these studies suggest that people with schizophrenia may have multiple abnormalities of the cholinergic system and that agents that enhance cholinergic function may act as cognitive enhancers.
The current 12-week placebo-controlled, double-blind, parallel-group, randomized clinical trial was designed to examine the efficacy and safety of galantamine for the treatment of cognitive impairments in people with schizophrenia.
Results
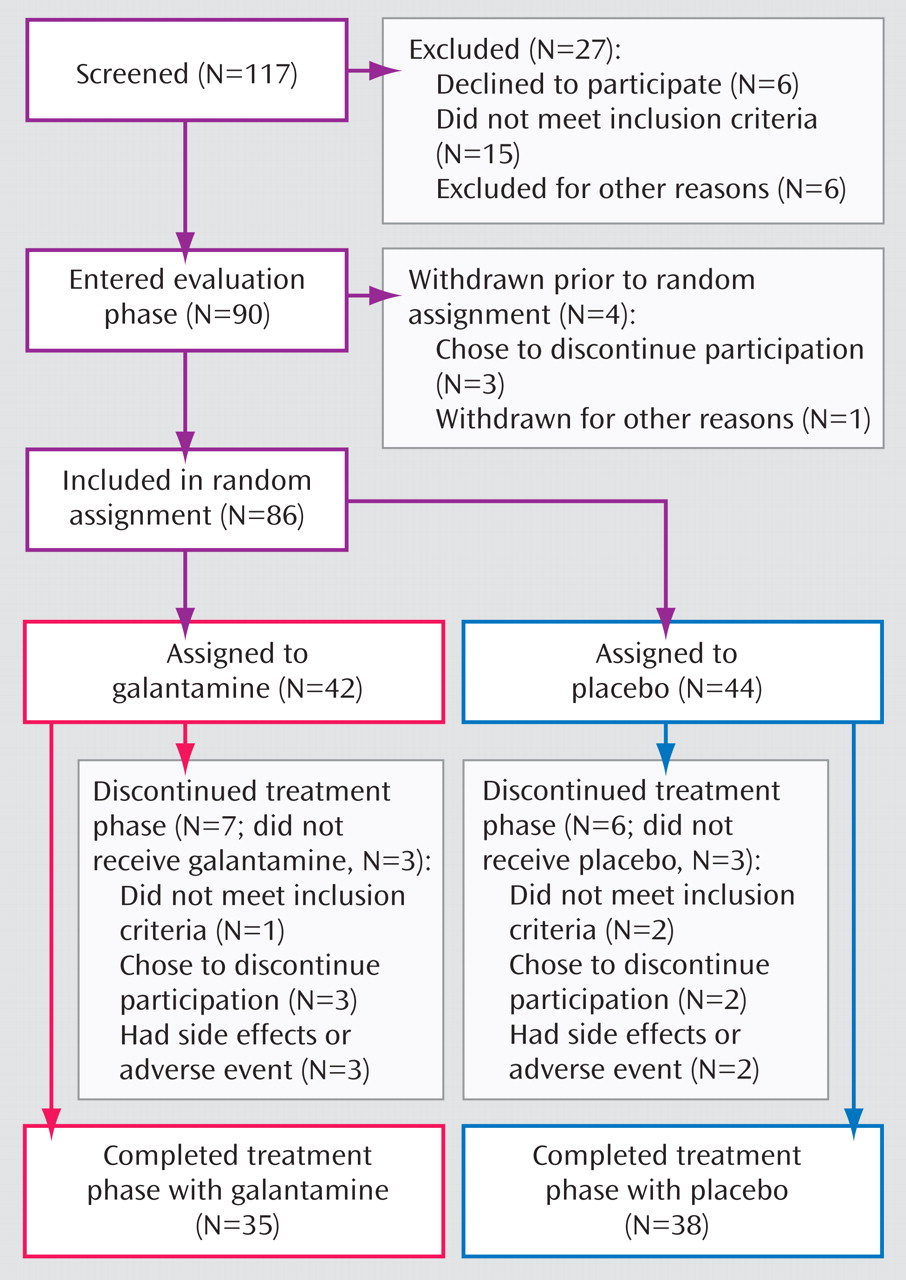
Subject flow is presented in
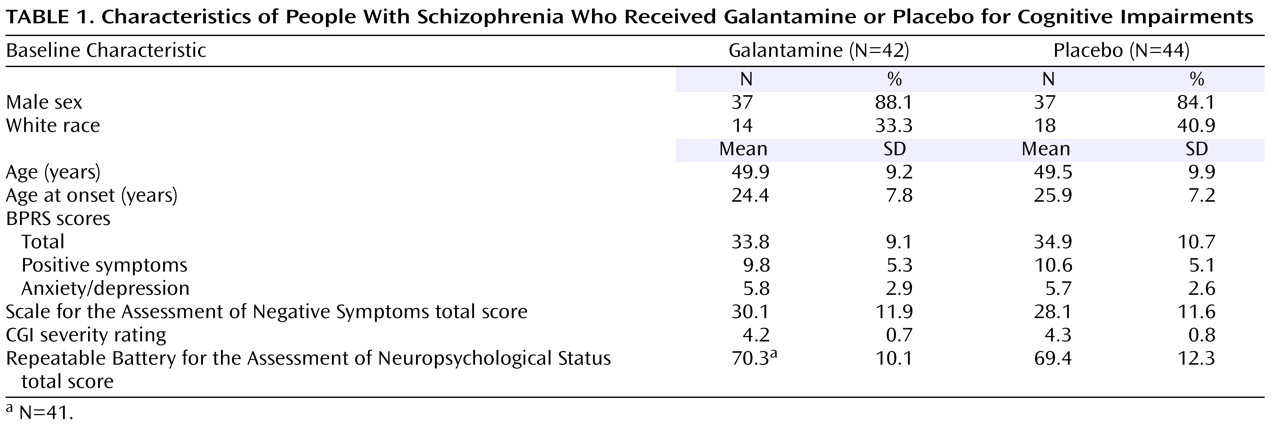
Figure 1 . Eighty-six subjects were randomly assigned to treatment. Thirty-two outpatients and 10 inpatients were assigned to galantamine, and 31 outpatients and 13 inpatients were assigned to placebo. Three subjects assigned to galantamine and three assigned to placebo were treated with low doses of conventional antipsychotics. The remaining subjects were treated with second-generation antipsychotics. Seventy-three subjects (35 taking galantamine and 38 taking placebo) completed the study. All of these subjects had valid baseline and end-of-study neuropsychological assessments and were included in the primary outcome analyses. Eighty subjects received either galantamine (N=39) or placebo (N=41) and were included in the safety analyses, and 79 subjects (39 taking galantamine and 40 taking placebo) received at least one postrandomization symptom assessment and were included in the secondary efficacy analyses. There were no significant differences in the demographic, clinical, or baseline symptom characteristics of the subjects who entered the double-blind phase (
Table 1 ).
Neuropsychological Measures
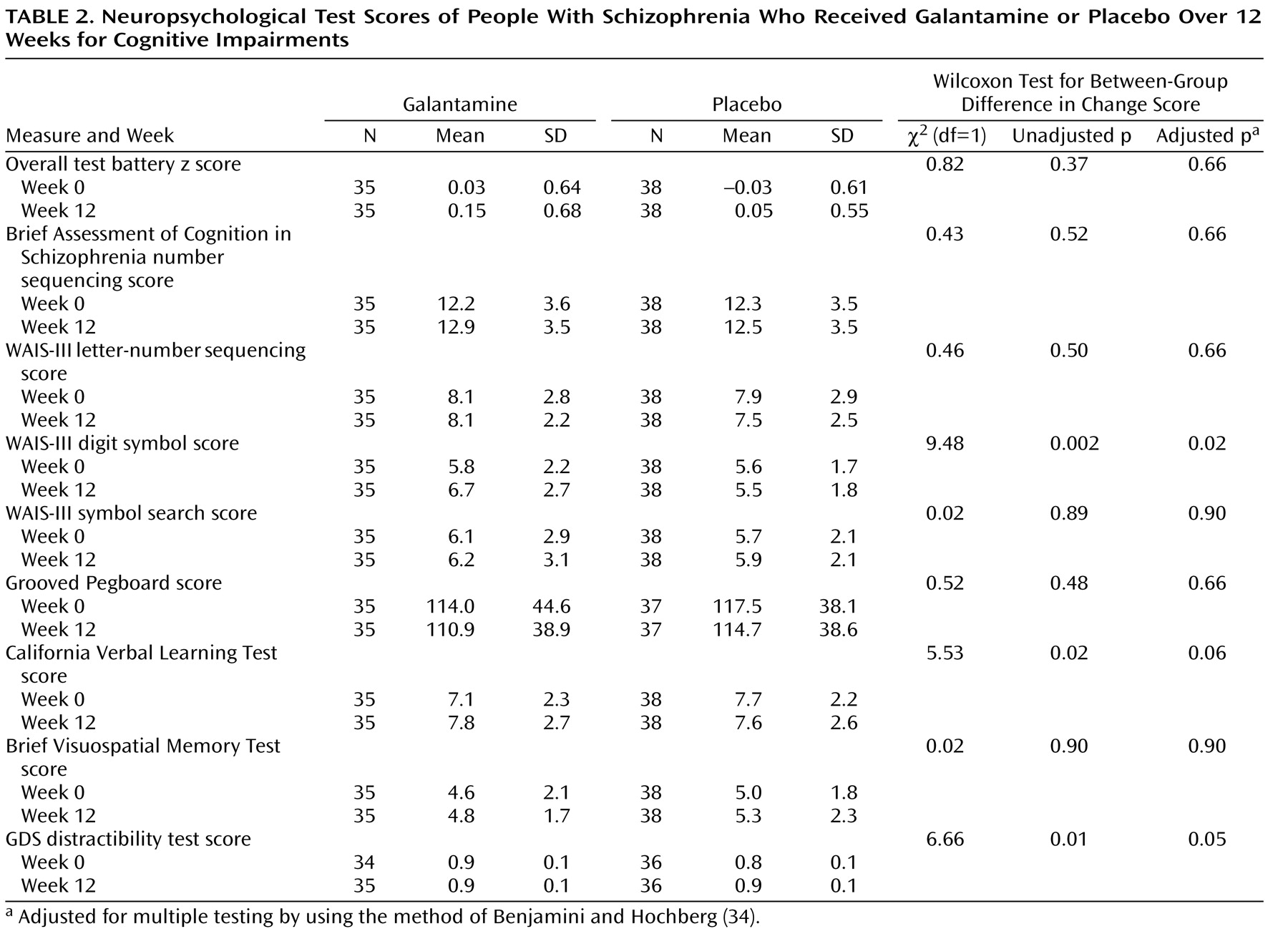
The mean baseline total scores on the Repeatable Battery for the Assessment of Neuropsychological Status for the subjects included in the analyses of neuropsychological test scores were 69.2 (SD=10.4) for galantamine and 67.5 (SD=11.1) for placebo (t=0.38, df=70, p=0.52). The mean galantamine dose at the end of the study was 23.5 mg/day (SD=2.7). The test z scores at 0 and 12 weeks for the composite and individual measures are presented in
Table 2 . The treatment effect for the composite score was not significant. However, the follow-up Pepe-Whitaker-Seidel test for heterogeneity of effect size for the individual test scores was significant (χ
2 =17.19, df=7, p=0.02), indicating that treatment effects were not uniform across individual measures. In the unadjusted analyses, there were three significant group differences: galantamine improved performance more than placebo on the WAIS-III digit symbol test and the California Verbal Learning Test, whereas placebo was more effective than galantamine for the GDS distractibility test (
Table 2 ). After adjustment of p values for multiple comparisons, the WAIS-III digit symbol and GDS distractibility test results remained significant, but the finding on the California Verbal Learning Test did not (
Table 2 ).
Fifteen of 35 galantamine subjects and 21 of 38 placebo subjects were classified as smokers at baseline. ANCOVAs for the interaction between smoking status and group assignment for the composite score and for each of the individual tests were not significant (in all cases, p>0.25).
Symptom Measures
The subjects had mild to moderate baseline levels of positive and negative symptoms (
Table 1 ). There were no significant group differences in the change in BPRS total score (F=0.16, df=1, 447, p=0.69), BPRS positive symptom item score (F=0.91, df=1, 447, p=0.34), or BPRS anxiety/depression factor score (F=0.71, df=1, 447, p=0.40). There was also no significant group difference in the change in SANS total score (F=2.76, df=1, 66.1, p=0.11). In exploratory analyses of the SANS subscales, there was a significant treatment group effect for the SANS alogia subscale, with galantamine subjects exhibiting a greater reduction in these symptoms (t=–2.77, df=71.2, p=0.007).
Safety Measures
There was a significant treatment group effect for the total score on the Simpson-Angus Rating Scale; for the galantamine group, the mean score fell from 1.4 (SD=1.8) at baseline to 1.1 (SD=1.8) at the end of the study, and for the placebo group the mean score was 1.6 (SD=2.1) at baseline and 1.6 (SD=1.9) at the end of the study (Conover-Salsburg test for treatment effect: t=2.03, df=77, p=0.05). The group treatment effect for AIMS total score was not significant. For galantamine the mean score was 2.0 (SD=3.1) at baseline and 2.1 (SD=3.0) at the end of the study, and for placebo the mean score was 2.1 (SD=3.1) at baseline and 1.6 (SD=3.0) at the end of the study (Conover-Salsburg test for treatment effect: t=0.35, df=77, p=0.73).
There were no significant group differences on the chemistry panel measures, including glucose, triglyceride, and cholesterol indices. The mean change in hemoglobin was significantly greater in the subjects assigned to galantamine (galantamine: mean change=0.29 g/dl, SD=0.69; placebo: mean change=–0.17, SD=1.06; t=2.19, df=72, p=0.03), with a similar but nonsignificant difference for hematocrit values (galantamine: mean change=0.81%, SD=2.17; placebo: mean change=–0.34, SD=2.98; t=1.89, df=72, p=0.06). There were no significant group results for the urinalysis, except for glucose. Three subjects receiving galantamine went from normal baseline values to abnormal values at the end of the study, whereas two subjects receiving placebo went from abnormal baseline values to normal values at the end of the study (χ 2 =7.06, df=3, p=0.05). All three of the galantamine subjects had a previous history of type II diabetes mellitus.
ECG results were remarkable for significant group differences in the mean change in PR interval (galantamine: mean change=25.7 msec, SD=56.0; placebo: mean change=–3.0, SD=12.0; t=3.00, df=71, p=0.004) and the mean change in the QRS interval (galantamine: mean change=11.4 msec, SD=25.1; placebo: mean change=–1.6, SD=11.7; t=2.86, df=72, p=0.006) but not the mean change in QTc interval (galantamine: mean change=3.03 msec, SD=28.89; placebo: mean change=3.03, SD=27.68; t=0.00, df=71, p=0.99).
There were no significant group differences on vital signs or on any of the items of the Side Effect Checklist, including nausea, vomiting, and change in weight. Three galantamine subjects withdrew because of adverse events. One subject developed pneumonia and was hospitalized, one subject had a worsening of positive symptoms and required adjustment of antipsychotic medications, and one subject resumed drinking alcohol. Two placebo subjects withdrew because of adverse events. One subject reported dizziness and attributed this to the study medication, and one subject had a worsening of positive symptoms.
Discussion
The study results suggest that galantamine does not exhibit significant global benefit for cognitive impairments in people with schizophrenia. Rather, galantamine appears to have a heterogeneous effect on neuropsychological test measures. Specifically, in unadjusted analyses galantamine was associated with improved performance on the WAIS-III digit symbol test and the California Verbal Learning Test. The treatment effect for the WAIS-III digit symbol test remained significant after correction for multiple comparisons. The effect on the California Verbal Learning Test was nonsignificant after adjustment but suggestive (i.e., p=0.06). In contrast, there was greater improvement on the GDS distractibility test in the subjects who received placebo. There was no effect of baseline smoking status on treatment outcome.
Galantamine had the most pronounced effect on WAIS-III digit symbol performance, a processing speed measure, although it had no effect on WAIS-III symbol search, another measure of processing speed. The digit symbol result is consistent with findings from two previous studies. Schubert and colleagues
(17) observed that, compared to placebo, galantamine had a significant effect on the attention index of the Repeatable Battery for the Assessment of Neuropsychological Status
(20), which comprises a digit symbol analogue and a digit span measure. Olincy and colleagues reported that acute challenge with DMXB-A also significantly improved performance on the attention index
(13) . We note that the digit symbol task requires cognitive and motor processes in addition to those needed for the symbol search, which suggests that the current results reflect the differential sensitivity of these two processing speed measures to treatment effects, rather than random error. Lending support to this conclusion is the finding from a recent meta-analysis, which compared the performance of people with schizophrenia and healthy comparison subjects on 37 neuropsychological measures, that the largest impairment was observed for digit symbol coding tasks
(37) .
Processing speed, as measured by digit symbol coding tasks, may be central to our understanding of the neuropsychology of schizophrenia. Not only do people with schizophrenia show severe impairment, but their relatives also show impaired performance on digit symbol tasks
(37) . Moreover, coding task performance is related to vocational outcome, independent living, and other expected community roles
(37,
38) . These studies suggest that measures of processing speed, particularly digit symbol coding tasks, are strongly predictive of functional outcome. In the current study, we did not include functional outcome or other coprimary outcome measures, so we could not directly address this issue. Future studies are required to test whether galantamine, with or without concomitant psychosocial intervention, is effective in improving community outcomes.
Galantamine appeared to improve verbal memory performance, although this effect showed greater interindividual variation than the digit symbol effect and was nonsignificant after adjustment for multiple comparisons. It is interesting that Schubert and colleagues also reported marked but variable improvement in immediate verbal memory
(17) . They did find significant improvement in delayed memory, which was not assessed in the current study. This combination of findings suggests that positive treatment effects on verbal memory may result for some subjects with galantamine treatment. Understanding the validity of such effects will require further study.
The GDS distractibility test was the only test for which there was a marked placebo effect. The significant performance improvement probably reflects a practice effect. The lack of similar improvement in distractibility for the galantamine group suggests the possibility that galantamine may block the practice effect on this measure. Partial support for this explanation is provided by the results of our open-label donepezil study, in which we found donepezil to have a similar adverse effect on GDS distractibility test performance
(39) . On the other hand, a large number of subjects in the current study performed near the test ceiling, which limits confidence in the finding. Additional study will be required to determine if the negative galantamine effect was an artifact or an unexpected but reliable result.
Galantamine had limited effects on symptoms. There was no evidence of an effect on positive or affective symptoms. Galantamine also did not result in global negative symptom improvement, but it did have a significant effect on the SANS alogia subscale. Future studies designed to specifically address the potential value of galantamine for negative symptoms are required.
Galantamine was well tolerated. Galantamine treatment was associated with a small but significant improvement in extrapyramidal symptoms. Galantamine also significantly increased hemoglobin levels, but not into the abnormal range. Finally, consistent with its known vagotonic properties, galantamine was associated with significant increases in the PR and QRS intervals, but not with prolongation of the QTc interval.
There have been a number of previous studies of acetylcholinesterase inhibitors in schizophrenia
(40) . Donepezil has been the most frequently tested agent. Although initial open-label trials and small controlled studies suggested a potential benefit
(40), a large multicenter study (N=245) failed conclusively to demonstrate any beneficial effects of donepezil on a comprehensive neuropsychological battery
(41) . Similarly, positive findings with open-label rivastigmine
(40) were not replicated in a double-blind, placebo-controlled study
(42) . Two small published double-blind studies examined the efficacy of galantamine for cognitive impairments in people with schizophrenia
(17,
18) . Schubert and colleagues (N=14) reported significant benefits of galantamine for the attention and delayed memory indices of the Repeatable Battery for the Assessment of Neuropsychological Status
(17) . Lee and colleagues (N=24) observed a significant improvement in visual recognition in galantamine-treated subjects
(18) . These subjects also showed nearly significant improvements in verbal recognition and attention, which may not have reached significance because of the small number of subjects
(18) . Finally, in an industry-sponsored study, there was no significant difference between galantamine and placebo on a global measure of cognition (clinicaltrials.gov, trial number: NCT00077727). The results for individual measures were not provided.
If the results of the Schubert, Lee, and current studies accurately reflect the cognitive benefits of galantamine, then the question arises of why galantamine has a beneficial effect but donepezil and rivastigmine apparently do not. In addition to acting as an acetylcholinesterase inhibitor, galantamine, in contrast to donepezil and rivastigmine, is also a positive allosteric modulator of the α
4 β
2 and α
7 nicotinic receptors
(14 –
16) . The allosteric properties of galantamine could lead directly to increased release of acetylcholine and activation of postsynaptic nicotinic receptors
(15) or act indirectly through its effects on the release of other neurotransmitters, especially glutamate and dopamine
(16,
43) . Schilström and colleagues found that galantamine increases dopaminergic activity and release in the prefrontal cortex in a dose-dependent manner
(16) . Wang and colleagues demonstrated that galantamine increased dopamine release in the hippocampus and that this effect was related to its ability to improve cognition in a mouse model of Alzheimer’s disease
(43) .
There are several potential limitations of the current study. First, the participants were relatively old. Second, the subjects’ mean total score on the Repeatable Battery for the Assessment of Neuropsychological Status was markedly lower than that in the study by Schubert et al.
(17) and suggests that the subjects were moderately cognitively impaired. These two factors may have limited the ability to detect the full range of benefit of galantamine treatment. A third potential limitation is the galantamine dose. Galantamine is an allosteric modulator at low concentrations, but at high concentrations the main effect of galantamine is mediated through its acetylcholinesterase inhibitor actions
(15) . In addition, at higher doses galantamine may act as an inhibitor of nicotinic receptors
(15) . In the current study, only one dose of galantamine was evaluated. If the cognition-enhancing effects of galantamine are mediated through its allosteric actions at the nicotinic receptors and if the current dose was above or below the maximum effective dose for these effects, then the observed results may underestimate the potential benefit of adjunctive galantamine treatment. Finally, there is the possibility that the acetylcholinesterase inhibitor activity of galantamine may have interfered with the potential benefits of its allosteric actions
(16) .
In summary, the current study results suggest that galantamine may have heterogeneous effects on cognitive performance. If the beneficial effects of galantamine are mediated through its allosteric actions at the nicotinic receptors, then future studies of more specific and potent allosteric modulators may be of considerable value.