Although the neurobiological basis of schizophrenia is not yet fully understood
(1), cross-sectional magnetic resonance imaging (MRI) studies in patients with schizophrenia have shown overall decreases in cortical gray and white matter volume and in smaller brain structures such as the amygdala-hippocampus complex, as well as volume increases in the lateral ventricles
(2,
3) . Moreover, the gray matter volume decrease and ventricular increase appear to progress over time with most
(4 –
12), but not all
(13), studies reporting the largest brain volume decreases in patients with the poorest outcome.
Of interest, poor clinical and functional outcome in schizophrenia has been consistently associated with cannabis use. Cannabis (ab)use occurs in 28%–50% of patients with schizophrenia
(14,
15), with those using cannabis having more positive
(16 –
20) (but not negative [
16,
18,
20 –
22 ]) symptoms, an earlier disease onset
(23), and an increased number of psychotic relapses or exacerbations
(18,
24,
25) compared to nonusing patients. Thus, if brain volume changes over time are most prominent in the patients with a poor outcome, and poor outcome is associated with cannabis use, one would expect cannabis use to be associated with excessive decreases in brain volumes. However, no longitudinal studies examining the effect of cannabis use on brain changes in schizophrenia have been conducted to date. In a cross-sectional study, we found that brain volumes in cannabis-using patients with recent-onset schizophrenia did not differ from those of cannabis-naive patients
(26) . This is consistent with the results of previous cross-sectional brain imaging studies comparing healthy subjects who use cannabis with those who do not (for a review, see reference
27 ). Brain volume changes over time, however, may prove to be more sensitive in detecting the effect of cannabis on the brains of schizophrenia patients. Indeed, cross-sectional MRI studies have failed to predict outcome over time
(28), whereas longitudinal MRI studies show that an excessive decrease in gray matter volume during the early course of the illness is associated with poorer functioning 2
(6) and 5
(29) years later. Therefore, we hypothesized that in view of the deleterious effects of cannabis on clinical outcome and the reported relationship between outcome and brain volume changes over time, cannabis-using patients with schizophrenia would show larger brain volume decreases over 5 years compared to nonusing patients and healthy comparison subjects.
Method
Subjects
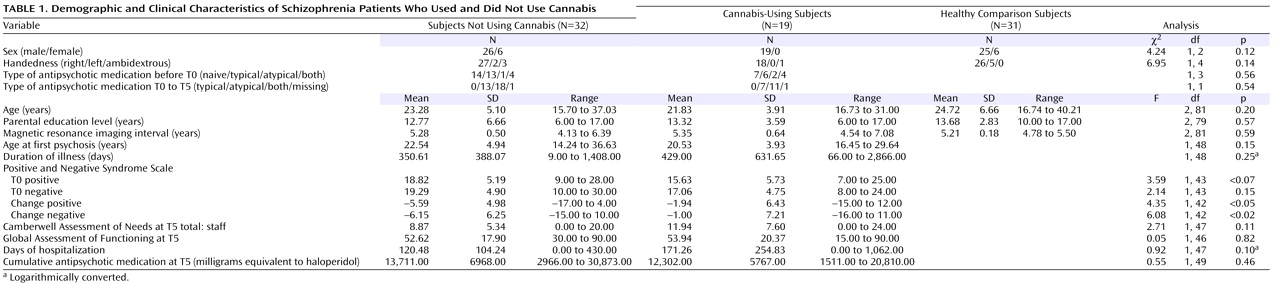
Patients with first-episode schizophrenia (N=51) and healthy comparison subjects (N=31) recruited from the First-Episode Schizophrenia Research Program at the University Medical Center Utrecht, Utrecht, the Netherlands, were included in the study. The cohort was followed for a period of 5 years to examine the relationship between brain morphology, the use of cannabis, and outcome. In this subgroup, patients and comparison subjects were included when two MRI scans were available with an interval of approximately 5 years. All patients met the DSM-IV criteria for schizophrenia and did not report drug use other than cannabis during the scan interval.
Both at baseline and at follow-up, the subjects were physically healthy and did not have a history of head injury. At inclusion, the patients were assessed with the Comprehensive Assessment of Symptoms and History
(30) by two trained raters who independently determined the diagnosis and achieved consensus afterward. All patients met the DSM-IV criteria for schizophrenia and did not report drug use other than cannabis during the scan interval. Severity of illness was measured with the Positive and Negative Syndrome Scale (PANSS)
(31) . Drug use was assessed with the Composite International Diagnostic Interview (CIDI)
(32), and the information provided by the patient was confirmed by a relative. Random urine toxicology tests were performed throughout the study. Patients with a lifetime diagnosis of abuse or dependence of a substance other than cannabis (except nicotine) were excluded. Five years later (T5), all 51 patients were reassessed for diagnosis (the Comprehensive Assessment of Symptoms and History), the need for care (the Camberwell Assessment of Needs
[33] ) and level of functioning (the Global Assessment of Functioning [GAF]), drug use (the CIDI), and symptom profile (the PANSS). The time interval between inclusion in the study at T0 and follow-up measurement at T5 is referred to as the scan interval.
At baseline (T0), 39 patients fulfilled DSM-IV criteria for schizophrenia, nine for schizophreniform disorder, one for schizoaffective disorder, and two for psychosis not otherwise specified; at T5, all 51 patients met DSM-IV criteria for schizophrenia. The number of days spent in the hospital between T0 and T5 was recorded. In addition, every patient was monitored carefully for the amount and type of medication prescribed between T0 and T5. Nineteen patients used only cannabis and no other illicit drugs during the scan interval, and 32 patients did not use any illicit drugs during the scan interval. Of this latter group, 15 patients never used cannabis during their lifetime, and 17 patients stopped using cannabis before baseline.
At follow-up, information was obtained on the average number of alcohol consumptions per week. Two patients in the cannabis-using group and two patients in the nonusing group met the DSM-IV criteria for alcohol abuse, whereas no healthy comparison subjects met these criteria. The three groups did not differ significantly on average number of alcohol consumptions per week at follow-up.
To calculate the cumulative dose of typical antipsychotic medication, a table from the Dutch National Health Service was used to derive haloperidol equivalents. The patients used only one antipsychotic at a time. For atypical antipsychotics, the respective pharmaceutical companies suggested how to convert the dose into haloperidol equivalents (clozapine, 40:1; olanzapine, 2.5:1; risperidone, 1:1; sulpiride, 170:1; quetiapine, 50:1; and sertindole, 2:1). The healthy comparison subjects were screened with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version
(34) and fulfilled criteria for “never mentally ill” both at baseline and follow-up. The groups were matched for sex, age, handedness, and socioeconomic status of their parents (expressed as the highest level of education completed by one of the parents). The healthy comparison subjects did not use any illicit substances before or during the study. After a complete description of the study to the subjects, written informed consent was obtained.
MRI Procedures and Measurements
MRIs were acquired on a Philips NT (Best, the Netherlands) scanner operating at 1.5 T for all subjects. A three-dimensional fast field echo (TE=4.6 msec, TR=30 msec, flip angle=30°, field of view=256×256 mm
2 ) scan with 160–180 contiguous coronal 1.2-mm slices and a T
2 -weighted dual-echo turbo spin echo (TE1=14 msec, TE2=80 msec, TR=6,350 msec, flip angle=90°, field of view=256×256 mm
2 ) scan with 120 contiguous coronal 1.6-mm slices of the whole head were used for the quantitative measurements. Processing was done on the neuroimaging computer network of the Department of Psychiatry at the University Medical Center Utrecht. Processing procedures have been described before
(35,
36) . In short, all images were coded to ensure investigator blindness to subject identification and diagnosis; scans were put into Talairach frames without scaling and corrected for inhomogeneities in the magnetic field. Quantitative assessments of intracranial, total brain, gray and white matter of the cerebrum, and lateral and third ventricle volumes were performed on the basis of histogram analyses and a series of mathematical morphology operators to connect all voxels of interest; they were validated previously
(37,
38) .
Statistical Analysis
Data were examined for outliers and normality of the distribution. Duration of hospitalization, illness duration, and lateral ventricle volume at T0 were not normally distributed, so these quantities were logarithmically transformed. No further transformation was performed on the data. To assess whether the groups differed for demographic or clinical variables, multiple analyses of variance were conducted for noncategorical variables and chi-square analyses for categorical variables (
Table 1 ).
To assess whether baseline brain volumes differed between patients with first-episode schizophrenia who used cannabis during the interval, patients with first-episode schizophrenia who did not use cannabis during the interval, and healthy comparison subjects, univariate analyses of covariance were performed with brain volumes at T0 as dependent variable and group (cannabis-using, non-cannabis-using, healthy comparison) as independent variable. Intracranial volume, age, and gender served as covariates.
Since we hypothesized that changes in tissue volume are proportional to baseline tissue volume, percentage volume change during the scan interval was calculated by dividing the absolute volume change by volume at baseline multiplied by 100% ([(T5–T0)/T0]×100%) for each individual.
To assess the difference in brain volume change between the groups, multiple general linear model univariate analyses of covariance were performed with percentage volume change as a dependent variable and group (cannabis-using, non-cannabis-using, healthy comparison) as an independent variable. Age at T0, gender, and intracranial volume served as covariates in all analyses. In case of significant findings, pairwise comparisons of main effects (least significant difference test) were performed.
To estimate clinical outcome in the different groups of patients, change in positive and negative symptoms over 5 years were quantified and baseline PANSS scores were subtracted from follow-up PANSS scores (PANSS T5 – PANSS T0).
In case of significant differences in volume change between the groups, we investigated the association between volume change and change in positive and negative symptom score between T0 and T5, score on the Camberwell Assessment of Need, and the GAF score at T5. All patients were included in multiple linear regression analyses with volume change as the dependent variable and clinical measure as the independent variable. Second, we repeated this analysis for each patient group separately.
Results
As shown in
Table 1, the groups did not significantly differ with regard to sex, handedness, age, parental education, and scan interval.
At inclusion and follow-up, the cannabis-using subjects and the subjects not using cannabis did not differ significantly on positive and negative symptoms and type and cumulative amount of medication during the interval. Cumulative duration of hospitalization did not differ significantly between the two patient groups. However, the subjects not using cannabis showed a small but significant improvement in positive and negative symptoms compared to the cannabis-using group over the follow-up interval. At inclusion, the cannabis-using and non-cannabis-using patients and healthy comparison subjects did not differ significantly on any of the brain volume measurements except for third ventricle volume at baseline, which was significantly larger in the non-cannabis-using group in relation to the healthy comparison subjects.
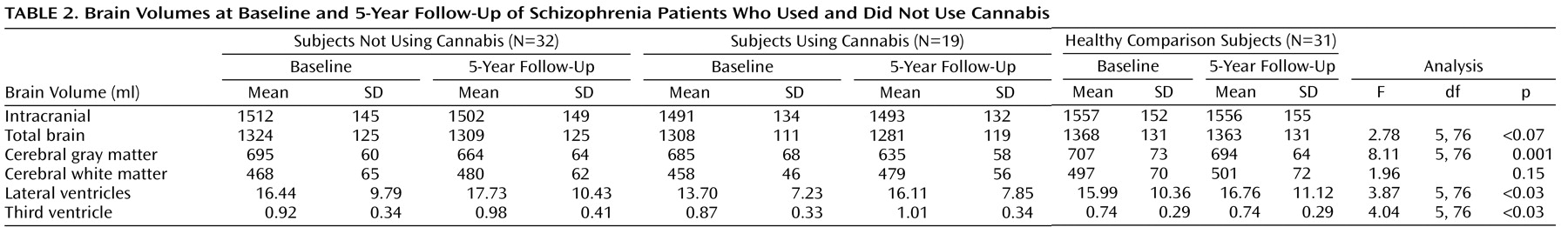
As shown in
Table 2, a significant main effect for group was found for change in cerebral gray matter (F=8.11, df=5, 76, p=0.001), lateral ventricle (F=3.87, df=5, 76, p<0.03), and third ventricle (F=4.04, df=5, 76, p<0.03) volume over the 5-year follow-up. A tendency was found for total brain volume change (F=2.78, df=5, 76, p<0.07). No significant group effect was found for white matter volume changes.
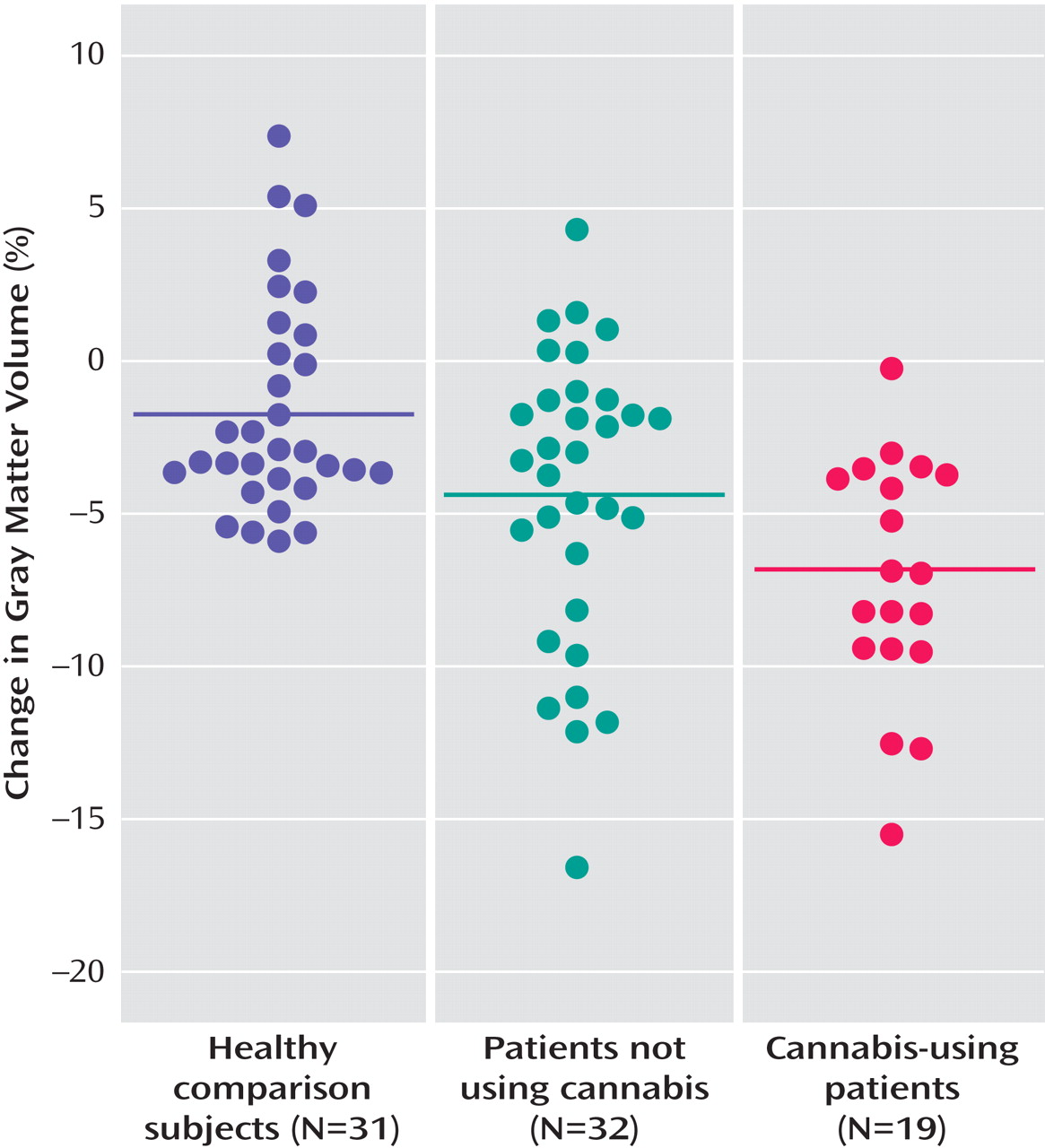
Pairwise comparisons showed significantly larger gray matter volume loss (
Figure 1 ) over time in cannabis-using patients in relation to healthy comparison subjects (mean difference=–5.09%; standard error=1.28%, p<0.001) and versus patients not using cannabis (mean difference=–2.67%; standard error=1.21%, p=0.03) and in non-cannabis-using patients in relation to healthy comparison subjects (mean difference=–2.42%; standard error=1.02%, p<0.03).
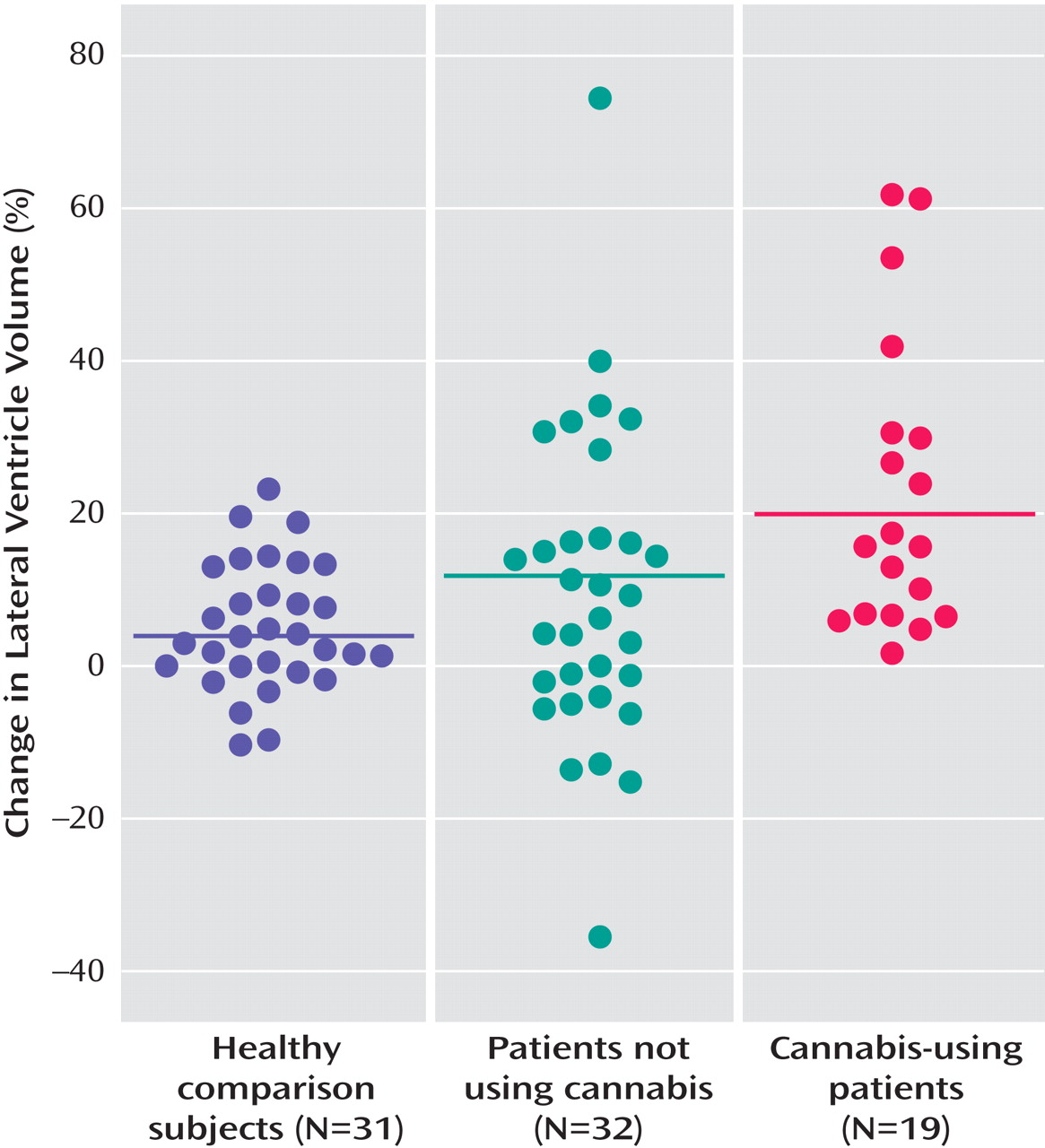
Furthermore, cannabis-using patients showed a more pronounced lateral ventricle enlargement (
Figure 2 ) over time in relation to healthy comparison subjects (mean difference=14.65%; standard error=5.34%, p=0.008) and compared to non-cannabis-using patients (mean difference=10.99%; standard error=5.05%, p<0.04).
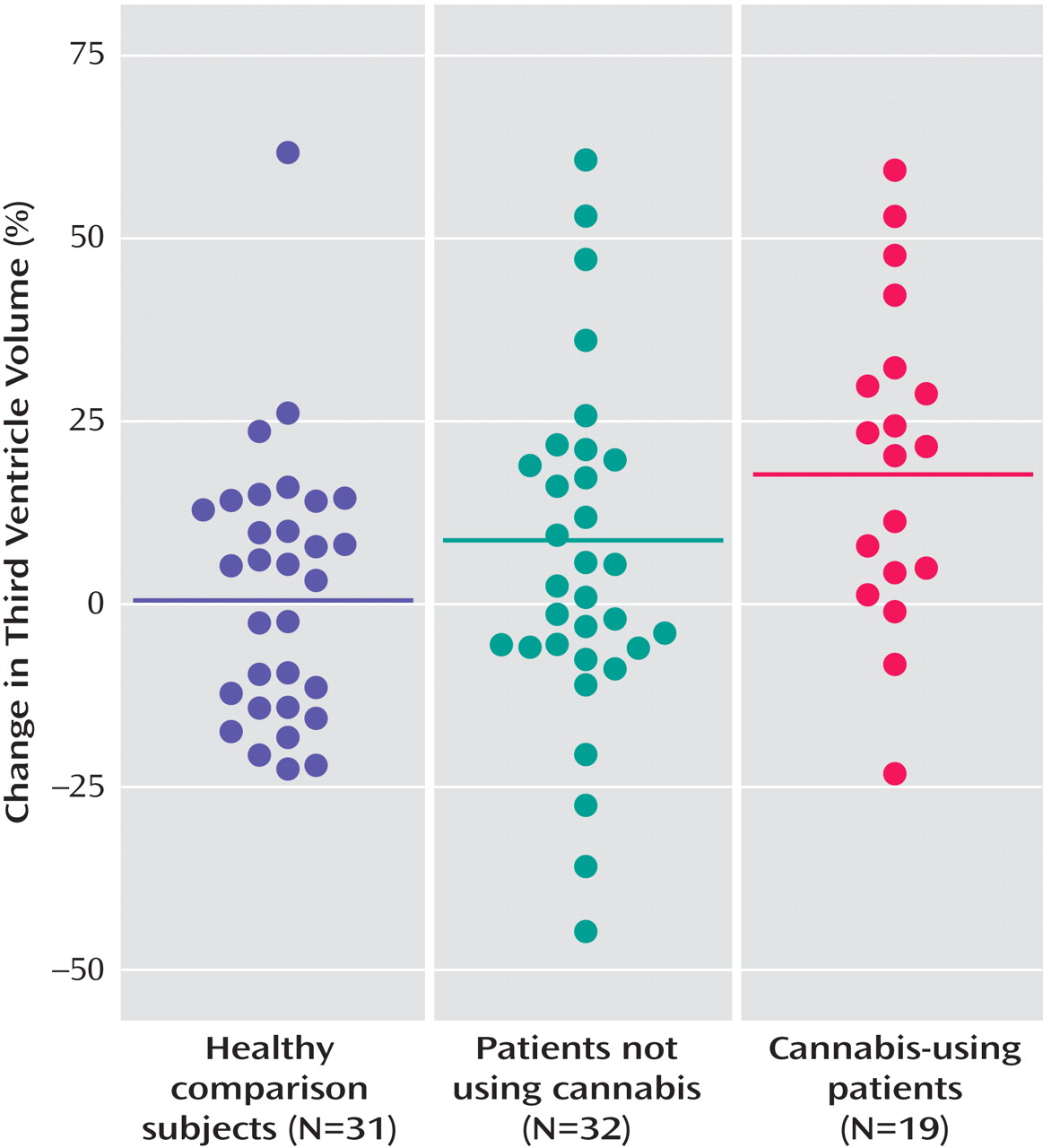
Cannabis-using patients also showed a more pronounced third ventricle increase (
Figure 3 ) in relation to healthy comparison subjects (mean difference=19.07%; standard error=6.87%, p=0.007) and patients not using cannabis (mean difference=14.89%; standard error=6.50%, p<0.03).
An increase in third and lateral ventricle volume during the interval was related to a higher Camberwell Assessment of Needs score; i.e., patients needed more help in daily life functioning (p<0.03 and p=0.006, respectively). Moreover, an increase in lateral ventricle volume was related to a lower GAF score (p=0.052). However, after dividing the patient group into cannabis-using and nonusing groups, no significant correlations with the clinical variables were present.
Discussion
This study finds that first-episode patients with schizophrenia who use cannabis show a more pronounced brain volume reduction over a 5-year follow-up (as expressed in lateral and third ventricle enlargement and gray matter loss) than patients with schizophrenia who do not use cannabis and compared to healthy subjects. Brain volume changes were measured over a 5-year interval in 19 first-episode schizophrenia patients who used cannabis during the interval, 32 first-episode schizophrenia patients who did not use cannabis during follow-up, and 31 cannabis-naive healthy subjects.
Finding progressive brain volume loss in schizophrenia patients, particularly of gray matter, is consistent with reports in high risk, first-episode, and chronic schizophrenia (for a review, see reference
39 ). Since most
(4 –
12), but not all
(13), studies find this decrease to be related to outcome, one could argue that the excessive decrease, as found in the cannabis-using group, is related to their slightly poorer outcome as they had less improvement in symptoms than nonusers. However, it is unlikely that the excessive brain volume loss in the cannabis-using patients can be wholly attributed to poorer outcome since global functional outcome measures and the amount of days hospitalized during the follow-up period did not significantly differ between the patients who used and those who did not use cannabis.
No significant correlations were present between brain volume change and measures of symptomatic and functional outcome in either of the patient groups. This could be due to the relatively small number of subjects in each group, which reduces the power to find significant associations.
Of importance, the excessive brain volume loss in the cannabis-using group could not be attributed to differences in baseline characteristics, such as brain volume or clinical measures. Indeed, as reported previously
(26), the cannabis-using group did not display larger brain volume abnormalities at onset compared to the nonusing group (nor versus healthy subjects). Similarly, although medication has been reported to affect brain volume loss over time
(5,
6,
11), the effects of medication cannot explain the excessive decrease in the cannabis-using patients since medication use over the follow-up period was qualitatively and quantitatively similar in both groups.
It could be argued that cannabis may amplify the preexistent vulnerability to brain volume changes associated with schizophrenia. The mechanism by which cannabis may cause neuronal damage in schizophrenia patients remains unclear. It could either be a direct consequence of cannabis intake or occur as a consequence of psychotic symptoms in schizophrenia that are associated with cannabis use
(16 –
20) . Indeed, it has been suggested that brain changes in the early stages of schizophrenia are the result of the “toxic” effect of the psychotic state
(8) . This would be consistent with our finding that the excessive brain volume decrease in the patients who continued to use cannabis was associated with less improvement in psychotic symptoms.
Since antipsychotic medication, especially atypical antipsychotic medication, has been suggested to attenuate the progressive brain changes in schizophrenia
(5,
6,
8,
11), excessive brain volume loss could also have been caused by noncompliance to antipsychotic medication in the cannabis-using patients. Indeed, it has been shown that patients who use cannabis are less compliant to prescribed antipsychotic medication
(40,
41) . Of interest, our group showed more negative symptoms compared to those reported in an earlier longitudinal study by Grech et al.
(18) . One would expect that patients with more prominent negative symptoms and poor social skills are less capable of gaining access to illicit drugs. However, in the Netherlands, personal use of cannabis is not illegal. Therefore, it is relatively easy for patients, even for those with more prominent negative symptoms and more impaired social skills, to have access to the drug. For the same reason, patients might be more likely to provide reliable information about drug intake.
Several limitations of this study should be taken into consideration. First, the number of patients included was limited. Second, we did not include a healthy comparison group using cannabis. However, several cross-sectional studies have failed to demonstrate an association between cannabis use and brain volume loss in healthy subjects (for a review, see reference
27 ). Third, the amount of cannabis used was estimated by the patients and their relatives. Although we are confident about whether a subject used or did not use cannabis, it is difficult to quantify the exposure because of differences in tetrahydrocannabinol (THC) content in cannabis cigarettes. Therefore, no information can be provided regarding a dose-response relationship between THC intake and brain volume change. Moreover, our study could not address the issue of direction of causality. In other words, it remains unclear whether brain volume loss results in a greater risk for using cannabis or whether continuous use of cannabis leads to excessive brain volume loss. Finally, owing to limited statistical power, we did not correct for multiple comparisons.
In conclusion, this study found a progressive gray matter decrease in schizophrenia during the first 5 years of the illness, which was more pronounced in patients who continued using cannabis after illness onset compared to patients and healthy subjects who did not use cannabis during the follow-up period. The patients who continued to use cannabis showed a less pronounced improvement in positive and negative symptoms compared to nonusing patients. Although further studies need to be conducted to confirm whether the brain volume loss is a direct or an indirect effect of cannabis in schizophrenia, this study suggests that some of the detrimental effects of cannabis on the course of illness may be explained by its effect on the progression of brain changes in schizophrenia.