Discussion
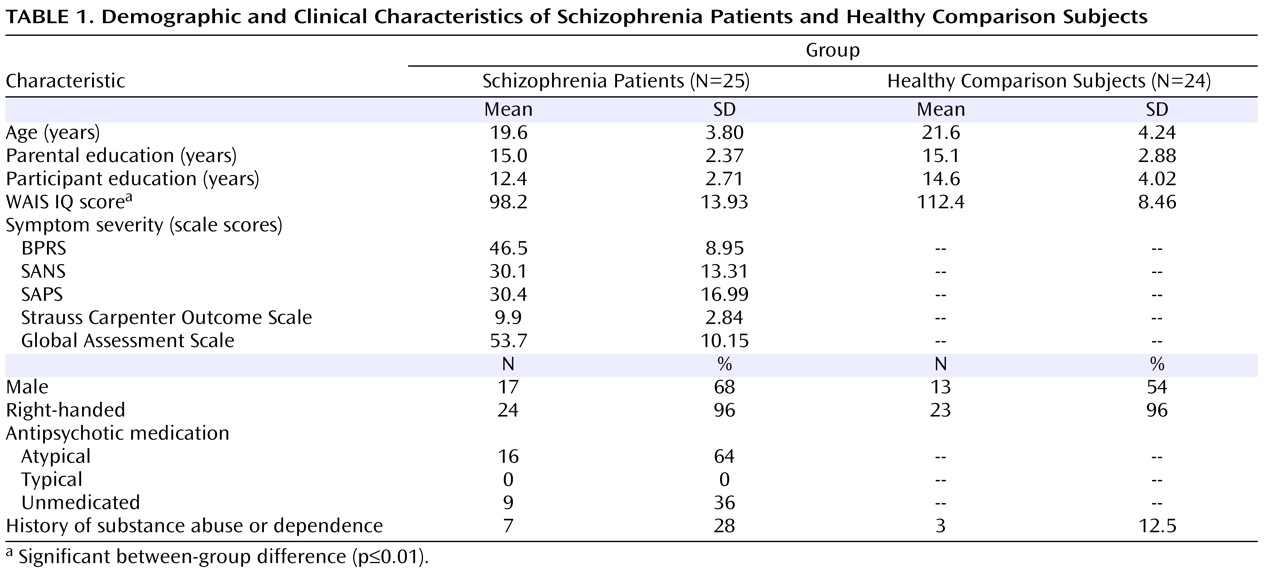
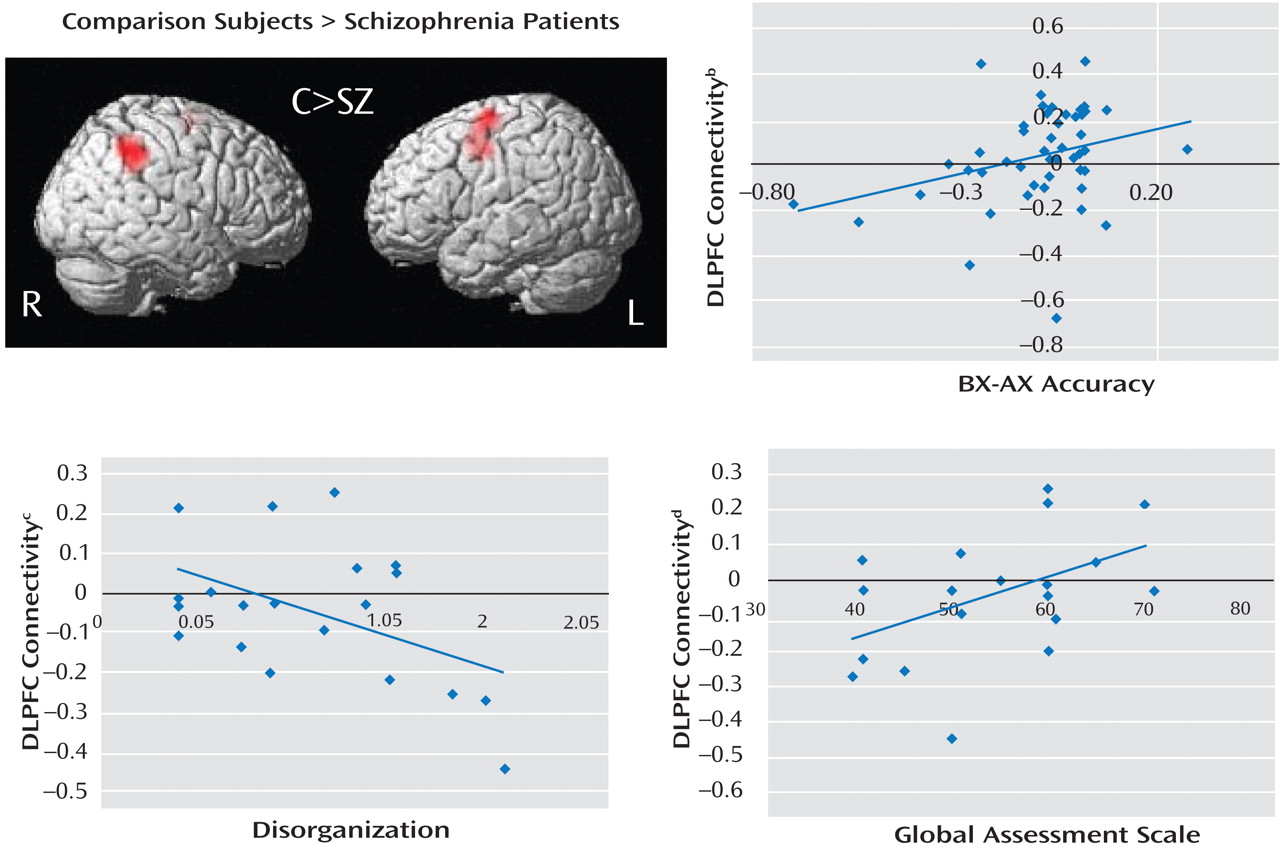
In a large sample of subjects with first-episode schizophrenia, we demonstrated a specific deficit in cognitive control, dysfunction of the dorsolateral prefrontal cortex, and significant correlations between impaired dorsolateral prefrontal cortex functional connectivity and behavior and clinical status. Schizophrenia patients exhibited poor performance during the BX condition, a condition that requires a high degree of cognitive control. However, patients did not differ from healthy comparison subjects in the AY and BY trials
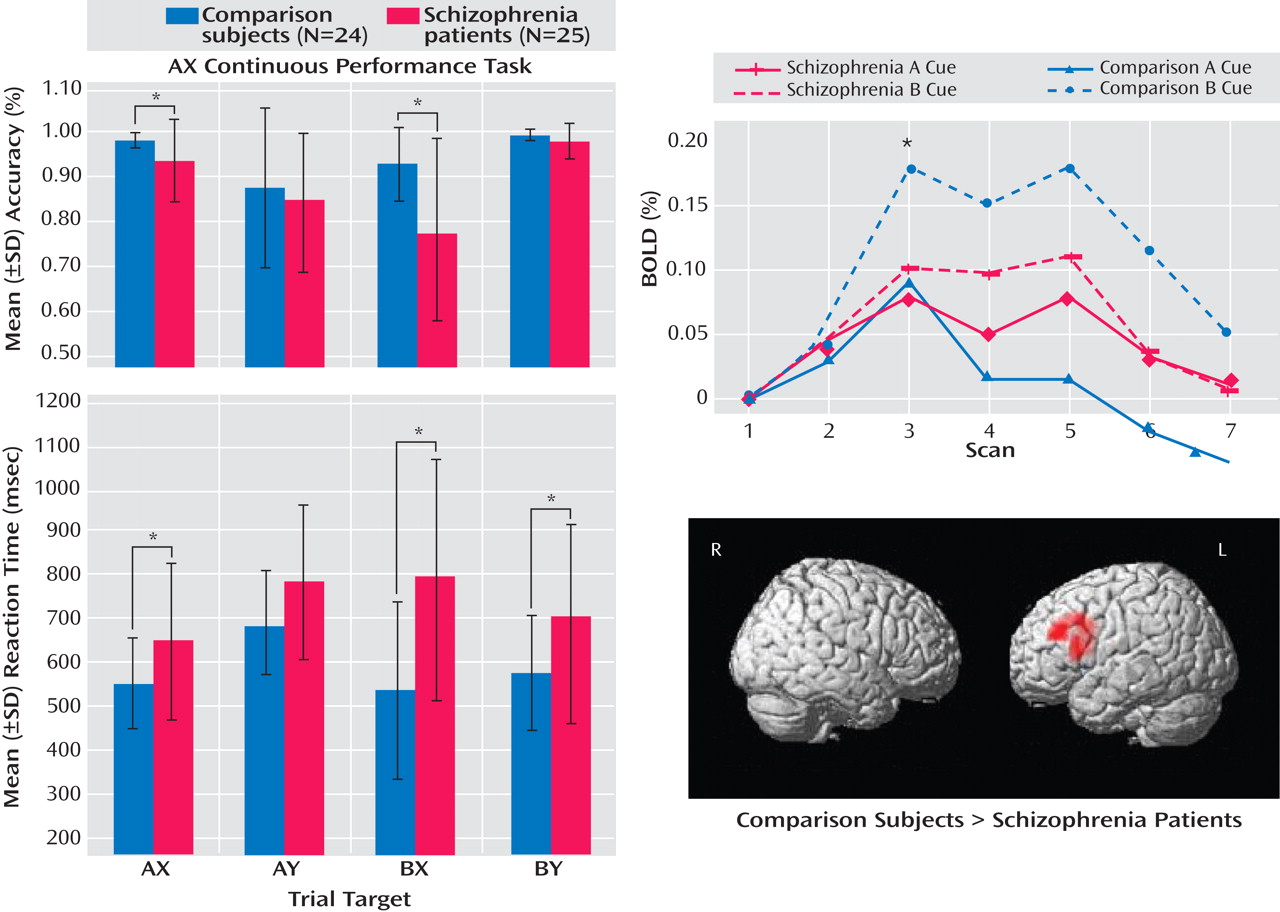
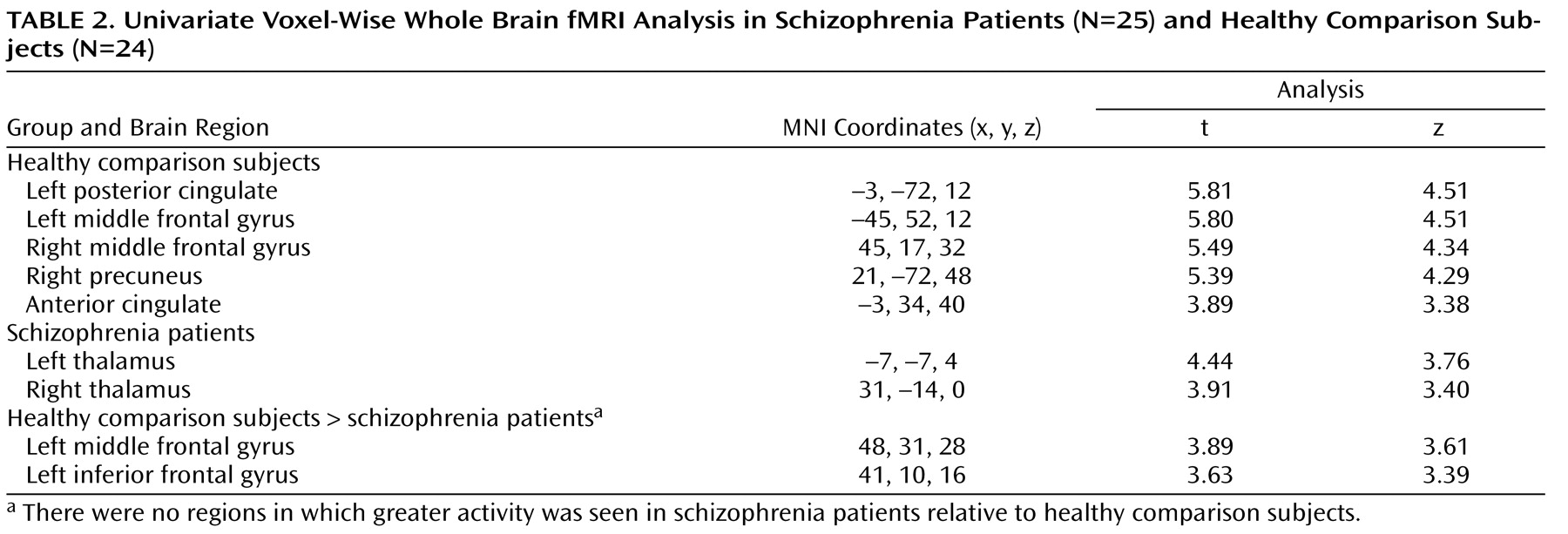
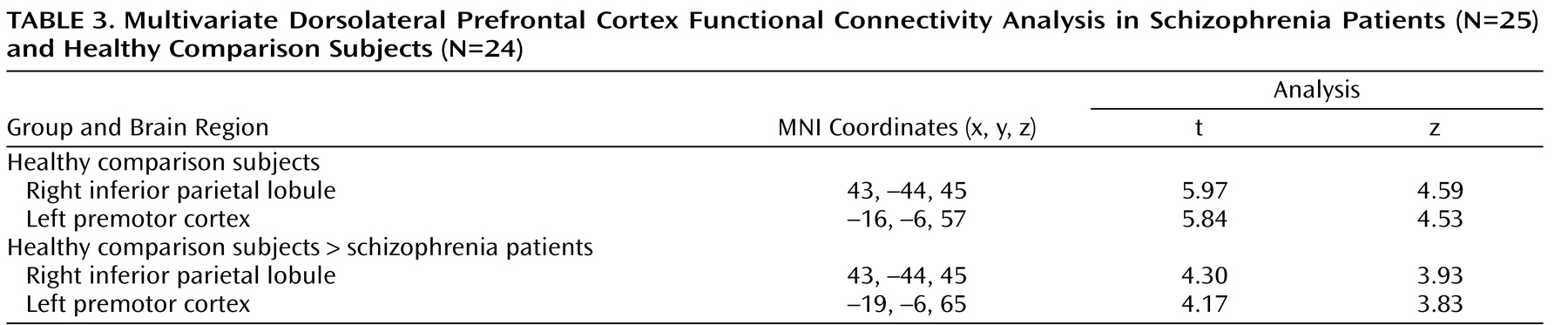
(12) . Schizophrenia patients were unable to up-regulate activity in the dorsolateral prefrontal cortex during B cues, as indicated by the results of the following two separate lines of analyses: BOLD time series derived from an a priori-identified dorsolateral prefrontal cortex region of interest and whole brain voxel-wise regression analysis. Additionally, there was a significant reduction in dorsolateral prefrontal cortex connectivity in the schizophrenia group, which suggests a deficit in dorsolateral prefrontal cortex top-down modulation. Across all subjects, we found significant correlations between the magnitude of dorsolateral prefrontal cortex connectivity and behavioral performance during the AX continuous performance task. In schizophrenia patients, we found significant correlations between connectivity and clinical measures (a positive correlation with GAS scores and an inverse correlation with disorganization). Lower dorsolateral prefrontal cortex connectivity was associated with worse performance, greater disorganization, and lower global functioning. To our knowledge, this is the first study to report an association between dorsolateral prefrontal cortex dysfunction and global measures of impairment.
The present study was motivated by empirical and theoretical research supporting the central involvement of the dorsolateral prefrontal cortex in cognitive control
(2 –
4) . Miller and Cohen proposed a model in which the dorsolateral prefrontal cortex biases activity in the posterior cortex, which is necessary for controlled performance
(3) . For example, during the delay period of a face working memory task, it is thought that the prefrontal cortex up-regulates the activity of face processing regions in the visual cortex when the neural representation of the to-be-remembered face must be maintained
(18,
19) . Recent animal and human studies have reported results that are consistent with this finding by demonstrating that lateral prefrontal cortex activity precedes and presumably guides the parietal region during controlled processing
(20,
21) .
A logical extension of this line of reasoning would be to propose that the magnitude of top-down modulation is a function of control demands. In the AX continuous performance task, we predicted that B cues would engage the dorsolateral prefrontal cortex in healthy comparison subjects to support task-appropriate processing and reduce BX error rates through top-down modulation. We examined this process using the beta correlation method combined with event-related analysis, which allows measurement of functional connectivity during discrete stages of task performance. Enhanced functional connectivity during the B cue condition relative to the A cue condition was observed in healthy comparison subjects, while there was a marked inability to enhance dorsolateral prefrontal cortex connectivity in schizophrenia patients. Previous studies have also shown altered interactions between cortical regions, suggesting the presence of disordered connectivity in schizophrenia
(22 –
25) . Accordingly, not only are our results consistent with the hypothesis that connectivity is impaired in individuals with schizophrenia, but they suggest that there is a potential fundamental neural mechanism by which dorsolateral prefrontal cortex dysfunction leads to cognitive impairment (i.e., top-down modulation through impaired connectivity with the dorsolateral prefrontal cortex).
As a measure of covariance, the beta series correlation method cannot determine the directionality of interactions. However, the fact that 1) only the dorsolateral prefrontal cortex (not other elements of the task-related neural network) showed differential activity in the univariate contrast in schizophrenia patients and healthy comparison subjects and 2) logistic regression analysis selectively associated decreased dorsolateral prefrontal cortex activity but not right inferior parietal lobule activity with a schizophrenia diagnosis provides converging support for our model. However, we cannot rule out the possibility that altered function in another element of the distributed network, such as the right inferior parietal lobule, underlies the altered pattern of connectivity. Future studies using tasks that selectively engage prefrontal and parietal cognitive control functions or other methods capable of detecting directionality of effects are needed to address this issue in a more definitive manner.
There may be concerns that our functional connectivity results were confounded by decreased dorsolateral prefrontal cortex activity in schizophrenia patients. However, the most important parameter constraining dorsolateral prefrontal cortex connectivity is not its mean activity but rather the magnitude of trial-to-trial variability. In fMRI, these are largely independent parameters, and lower dorsolateral prefrontal cortex connectivity in patients is not necessarily the result of low univariate activity. This was verified in our data by the demonstration of equivalent dorsolateral prefrontal cortex variance between groups. Other examples of the divergence between the mean univariate and functional connectivity properties of a region are present in the extant literature
(17) .
In the present study, we included adolescent subjects in order to ensure that we investigated a representative sample of first-episode schizophrenia patients
(26) . In this study and a previous report
(27), we closely matched the study groups on age to ensure that there were no developmental differences, aside from those that may be related to the illness, between the groups. Further, in a reanalysis of our data in the present study, which excluded subjects <16 years old, we found an identical pattern of results. To address the possibility that lower dorsolateral prefrontal cortex activity in schizophrenia patients might have been the result of lower accuracy on the AX continuous performance task among these individuals (and hence a lower number of correct BX trials), we conducted an analysis in which only performance-matched subjects were included. This analysis yielded fMRI results that were identical to those from the full sample.
An examination of the putative functions of cortical regions identified in the dorsolateral prefrontal cortex connectivity analysis revealed that each of these regions may support task-relevant processes during the AX continuous performance task. The right inferior parietal lobule has strong anatomical connections to the dorsolateral prefrontal cortex
(28), and recent functional neuroimaging studies in humans suggest that the right inferior parietal lobule supports sustained attention
(29,
30) . Similarly, the frontal eye field is thought to support visual attention, in addition to its well-recognized role in eye-movement motor control
(31,
32) . It is possible that functional impairment in one of these regions could lead to disrupted connectivity with the dorsolateral prefrontal cortex.
Limitations to the present study include the inferences that could be made pertaining to the relative importance of brain regions other than the dorsolateral prefrontal cortex
(33 –
35) in the broader pathophysiology of schizophrenia. Although the demonstration of a significant association between dorsolateral prefrontal cortex connectivity and behavioral and clinical impairments (particularly with disorganization symptoms and GAS) suggests broad relevance of dorsolateral prefrontal cortex dysfunction, the determination of the relative importance of the dorsolateral prefrontal cortex compared with other regions involving other cognitive processes needs to be examined in future studies.
Other possible limitations are the effects of medication on fMRI measures. The available evidence argues against medication effects as a major contributor to our findings. Prior studies have shown that antipsychotic drugs do not suppress the prefrontal cortex BOLD signal during higher-order cognition
(27,
36) . In the present study, there were no differences between medicated and unmedicated patients in cue-related dorsolateral prefrontal cortex activity. Furthermore, since our patients were very early in the course of illness, it is reasonable to conclude that impaired dorsolateral prefrontal cortex function is fundamental and unrelated to the long-term effects of medication exposure or illness chronicity.
Although the physiological mechanisms underlying impaired dorsolateral prefrontal cortex activity are unknown, the asynchronous activity of neurons as the result of perturbations in the local circuitry is a cogent potential mechanism
(37,
38) . Simultaneous recording and fMRI studies in primates suggest that synchronous firing of cortical neurons, particularly in the high-frequency range (40–80 Hz or gamma), is highly correlated with BOLD response
(39) . In a recent EEG study, which parallels the present fMRI study, we reported a decrease in induced gamma-band activity associated with impaired cognitive control performance and disorganization in schizophrenia patients
(40) . The results of the present study are consistent with the findings of our previous EEG study and with a model of impaired synchronous neuronal firing in the dorsolateral prefrontal cortex, which is associated with disruption in top-down-regulation of neural networks that support cognitive control.
The convergence of behavioral, functional neuroimaging, and clinical findings in the present study supports the dorsolateral prefrontal cortex dysfunction model of cognitive control deficits in schizophrenia. Furthermore, the reported functional connectivity results provide new insights into how dorsolateral prefrontal cortex dysfunction may lead to impaired cognitive control, behavioral disorganization, and functional impairment in individuals with schizophrenia by suggesting a physiological model in which a failure to recruit and maintain a coordinated network may underlie the cognitive impairment and related aspects of the psychopathology of schizophrenia.