Safe and effective treatments are needed for youths, but there is a paucity of double-blind and placebo-controlled treatment studies in adolescents with schizophrenia. In a 4-week study of 75 adolescents with schizophrenia between the ages of 13 and 18 years, treatment with either loxapine or haloperidol was reported to be superior to placebo in reducing symptoms of psychosis
(10) . The most common side effects reported in that trial were extrapyramidal symptoms and sedation. In an 8-week study in which 107 adolescents between the ages of 13 and 17 years were randomly assigned in a 2:1 ratio to receive either olanzapine or placebo in a double-blind fashion, active treatment with olanzapine was associated with greater reductions in psychiatric symptoms than placebo
(11) . In that study, weight gain and sedation were reported to occur more commonly with active treatment than with placebo. The study also found elevations in cholesterol, triglycerides, glucose, insulin, and prolactin with active treatment when compared with placebo.
This randomized, double-blind, placebo-controlled clinical trial assessed the efficacy and tolerability of aripiprazole in the acute treatment of adolescents with schizophrenia. The a priori hypotheses were that aripiprazole would be superior to placebo in ameliorating symptoms of psychosis and would be generally well tolerated.
Method
Study Design
In accordance with the U.S. Code of Federal Regulations (CFR Title 21, Part 50), written, informed consent was obtained from all subjects’ guardians or legal representatives, and written assent was obtained from each adolescent subject before any study-related procedures were performed. The protocol, procedures, consent, and assent statements (per guidelines of the U.S. Food and Drug Administration [FDA] and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) were approved by the institutional review board or ethics committee of each participating center
(18,
19) .
This was a phase III, randomized, placebo-controlled study. The trial was conducted in 101 centers in the United States, Europe, South America, Asia, the Caribbean, and South Africa over a 23-month enrollment period. Randomization was stratified and equally maintained across each of three geographical categories: 1) United States, 2) Europe, and 3) other regions. To assure reliability of ratings among sites, the number of raters within each site was minimized and efforts were to be made to ensure that the same rater administered the Positive and Negative Syndrome Scale (PANSS)
(20,
21) for a given patient. Raters at each site had identical instructions for administering the PANSS, and each site was required to demonstrate interrater reliability on an ongoing basis throughout the period in which patients participated in the study.
Patients
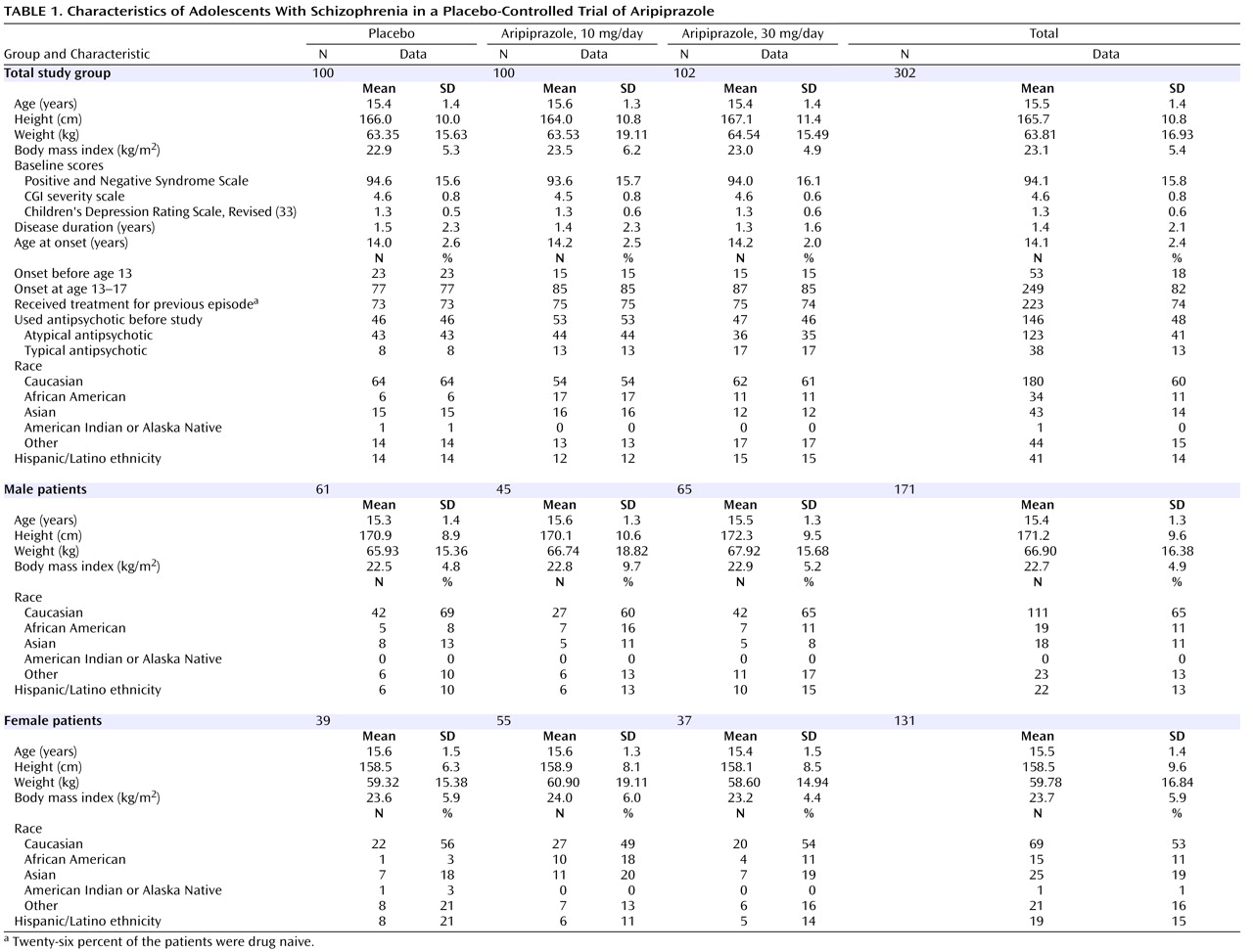
Subjects who were deemed appropriate candidates by their treating physicians were screened for eligibility within 4 weeks of baseline. Eligible subjects met the following inclusion criteria: 1) male or female, 2) age 13 to 17 years inclusive, 3) DSM-IV
(22) axis I primary diagnosis of schizophrenia and confirmation of the schizophrenia diagnosis by an adequately trained clinician (e.g., child psychiatrist) at the time of screening, by means of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL)
(23), and 4) a baseline PANSS score of 70 or higher. Eligible subjects were randomized in a 1:1:1 ratio to receive placebo, 10 mg/day of aripiprazole, or 30 mg/day of aripiprazole for 6 weeks. On the basis of an effective and well-tolerated dosing schedule used in previous sponsor-conducted trials
(24 –
26) and the FDA written request to examine the dose range of aripiprazole for adults
(12 –
15), eligible subjects received aripiprazole according to a forced-titration scheme to achieve target doses of either 10 or 30 mg/day. In the 10-mg group, aripiprazole was titrated from a starting dose of 2 mg/day to 5 mg/day on day 3, followed by an increase to the target dose of 10 mg/day on day 5. Subjects in the 30-mg group also began at 2 mg/day; the dose was then increased every 2 days to 5 mg/day, 10 mg/day, 15 mg/day, 20 mg/day, and finally, the target dose of 30 mg/day on day 11. Target doses were maintained for at least 2 weeks. Subjects who experienced unacceptable dose-related tolerability problems before study day 25 were removed from the study. After day 25, a dose reduction to a minimum of either 5 or 15 mg/day was permitted for subjects in the 10-mg and 30-mg cohorts, respectively. After dose reduction, a return to the initial target dose was not permitted. Medication was administered once daily.
Subjects were required to discontinue prohibited medications, including mood stabilizers, antidepressants, and other psychotropics, at least 3 days before the initiation of treatment. Fluoxetine must have been discontinued for 4 weeks, and stimulants and atomoxetine must have been discontinued for at least 5 half-lives before the first dose of study drug. During the washout period, benzodiazepines (lorazepam or equivalent) or anticholinergic agents were permitted as clinically indicated. During the course of the study, if the primary efficacy measure was unchanged or worsened, or if deemed absolutely necessary by the investigator, subjects were permitted to receive benzodiazepine or anticholinergic medications for relief of transient symptoms. Treatment with benzodiazepines within 4 hours or with anticholinergic agents within 12 hours before efficacy ratings or administration of movement scales was prohibited.
Subjects were excluded if they had any current psychiatric comorbidity requiring pharmacotherapy; any evidence of suicide risk; or a history or current diagnosis of schizoaffective disorder, mental retardation, major depressive episodes, neuroleptic malignant syndrome, any neurologic disorder other than Tourette’s syndrome, severe head trauma, or any unstable medical condition. Subjects whose illness was resistant to antipsychotics according to prior trials of two different antipsychotics of adequate dose and duration were also excluded, as were adolescent girls who were pregnant or breast-feeding and sexually active adolescent boys or adolescent girls who did not agree to abstinence or birth control. Subjects with positive screens for illegal drugs within 3 months of baseline or during the study were either excluded or removed from the study, respectively. Additionally, subjects who had been hospitalized for acute schizophrenia within 2 weeks or enrolled in a clinical trial within 4 weeks of baseline testing were excluded. Subjects were permitted to participate in this study on an outpatient, partial hospitalization, or full inpatient basis at any given time of the study.
Efficacy Assessments
The a priori primary efficacy assessment was the mean change from baseline to the last observed postbaseline visit (with the last observation carried forward) in total score on the PANSS. Assessments were performed at baseline and at weekly visits through day 42, which was the end of the study (week 6).
The secondary efficacy measures included the mean change in the PANSS positive and negative subscale scores from baseline to the last observation. The secondary measures also included the Clinical Global Impression (CGI) improvement and severity scales
(27) and the clinician-rated Children’s Global Assessment Scale
(28) . The latter instrument was completed at baseline and at day 42 or the end of treatment.
Quality of life and patient satisfaction were assessed by using the Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire
(29) . Both the “total score” and a separate “overall score” item were determined at screening and at day 42.
Safety and Tolerability Assessments
To avoid bias in responses about adverse events, the subjects (and their parents or guardians) were asked, “How have you felt since your last visit?” The investigators were required to record all spontaneously reported adverse events at all scheduled assessments.
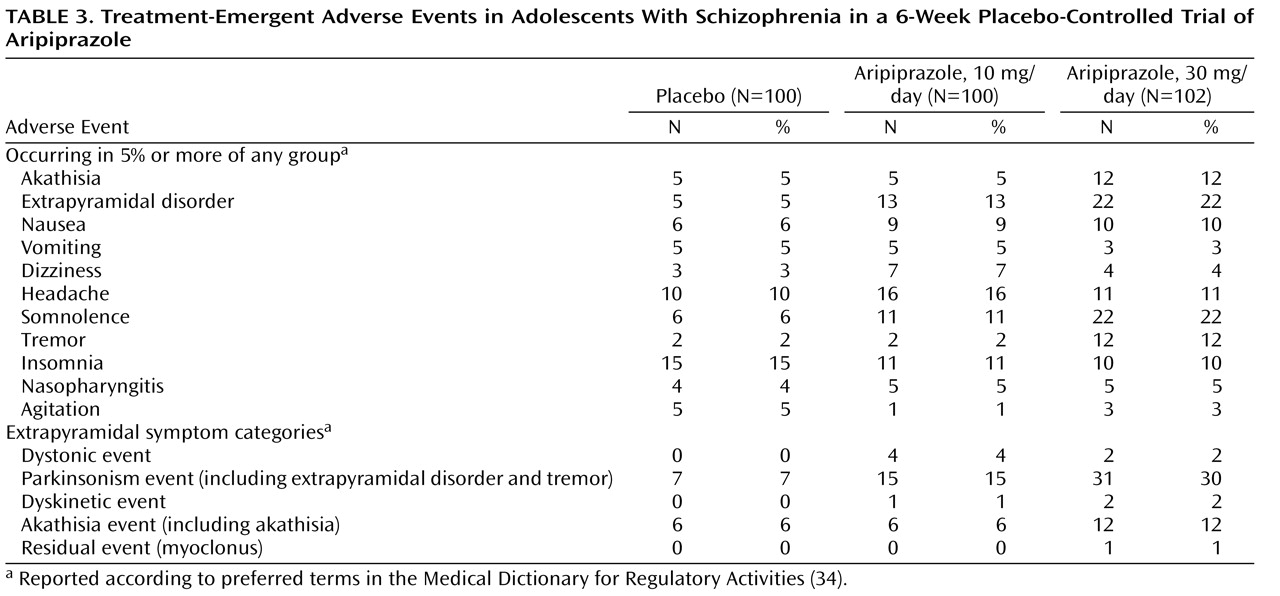
Side effects related to extrapyramidal symptoms were assessed at each visit as spontaneously reported adverse events. Extrapyramidal symptoms were also monitored at each scheduled visit by means of the Simpson-Angus Scale
(30), the Barnes Rating Scale for Drug-Induced Akathisia
(31), and the Abnormal Involuntary Movement Scale (AIMS)
(32) .
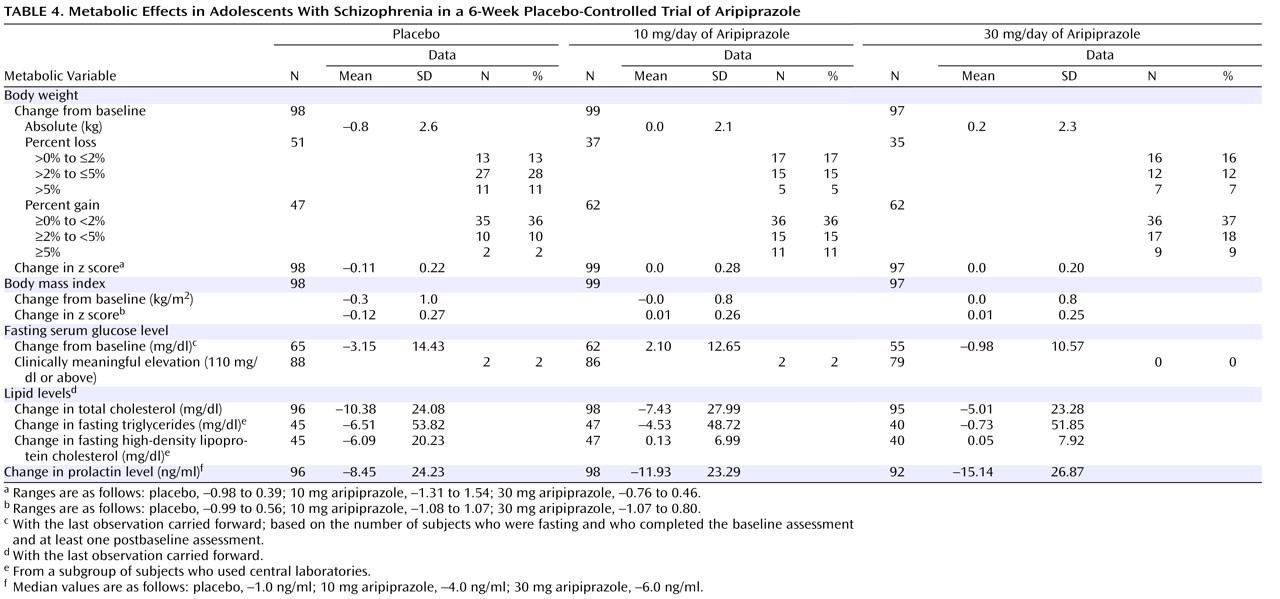
Metabolic evaluations included body weight, body mass index, diastolic and systolic blood pressure, and fasting levels of serum triglycerides, high-density lipoprotein (HDL) cholesterol, and glucose. Blood samples for measuring triglycerides, HDL cholesterol, and glucose were obtained after a minimum 8-hour fast at baseline, day 28, and day 42 (or end of study).
Additional assessments included ECG, physical examinations, vital signs, and number of hospitalizations. An independent five-member data safety monitoring board was informed of adverse events throughout the course of the study for the purpose of ensuring patient safety.
Statistical Analyses
Using a two-sided alpha at the 0.025 level of significance, we determined that approximately 255 subjects (85 subjects per treatment arm) were required to provide at least an 85% power to detect a between-group difference of –11.4 in the mean change from baseline in the primary efficacy assessment (PANSS total score). This study group size was based on the assumption that variability in this assessment would be similar to that in adult schizophrenia trials over the 2- to 10-mg/day and 2- to 30-mg/day ranges, which resulted in a –11.4 median of the mean differences with a pooled standard deviation of 22.5
(12 –
15) . Enrollees without a baseline or a postbaseline PANSS value were not included in the primary analysis of change from baseline in PANSS total score.
Statistical analysis was performed by fitting an analysis of covariance (ANCOVA) model to the PANSS change scores with right-hand terms for treatment, regional strata as factors, and baseline total score as covariate. The least-squares means derived from this model were used for comparing placebo with each active treatment. A nominal overall significance level of 0.05 (two-tailed) was used in testing the statistical significance of these two comparisons. The Hochberg’s procedure was used to account for multiplicity in testing the two comparisons, with the following criteria: if both p values were <0.05 (two-tailed), then statistical significance was declared for both doses; if the larger of the two p values was >0.05, then the smaller p value was compared with 0.025 (two-tailed). The corresponding treatment comparison was declared statistically significant if the p value was <0.025.
The data set at the day 42 visit, with the last observation carried forward, was used for the primary efficacy analysis. Scores on the Children’s Global Assessment Scale, CGI severity scale, and PANSS positive and negative subscales were analyzed by fitting an ANCOVA model to the change scores with treatment, regional strata, and baseline value as right-hand terms. The same ANCOVA model was applied to the change in scores on the Simpson-Angus Scale, the Barnes Rating Scale for Drug-Induced Akathisia, and the AIMS. Scores on the CGI improvement scale and the overall score on the Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire were analyzed by the Cochran-Mantel-Haenszel method, with regional strata as the stratification factor. Kaplan-Meier curves were plotted, and the log-rank test was used to test the significance of the difference in survival curves for time to discontinuation due to all reasons. Remission was defined as a score of 3 or less for items P1, P2, P3, N1, N4, N6, G5, and G9 of the PANSS, and the between-group differences in remission rate were analyzed by means of the Cochran-Mantel-Haenszel method, with regional strata as the stratification factor. The primary data set for analysis of scores on the Children’s Global Assessment Scale, CGI severity and improvement scales, and PANSS positive and negative subscales was the last-observation-carried-forward data set at the day 42 visit. Both observed cases and the last observation carried forward were analyzed at all other visits. A significance level of 0.05 (two-tailed) was used for all secondary efficacy analyses to declare statistical significance. Analysis of change from baseline in PANSS total score was performed both for observed cases and the last observation carried forward at all visits before the day 42 visit, by means of methods similar to those already described.
Additionally, the patient dropout rates for each aripiprazole group and the placebo group were compared by using the Cochran-Mantel-Haenszel method stratified by regional strata. Statistical comparisons of mean changes in tolerability measures (e.g., extrapyramidal symptoms, metabolic variables, and ECG measures [QTcB interval]) yielding p values were performed by ANCOVA models on the respective change scores. The p values for comparing time to discontinuation from the study in each aripiprazole group and the placebo group were obtained by the log-rank test.
Discussion
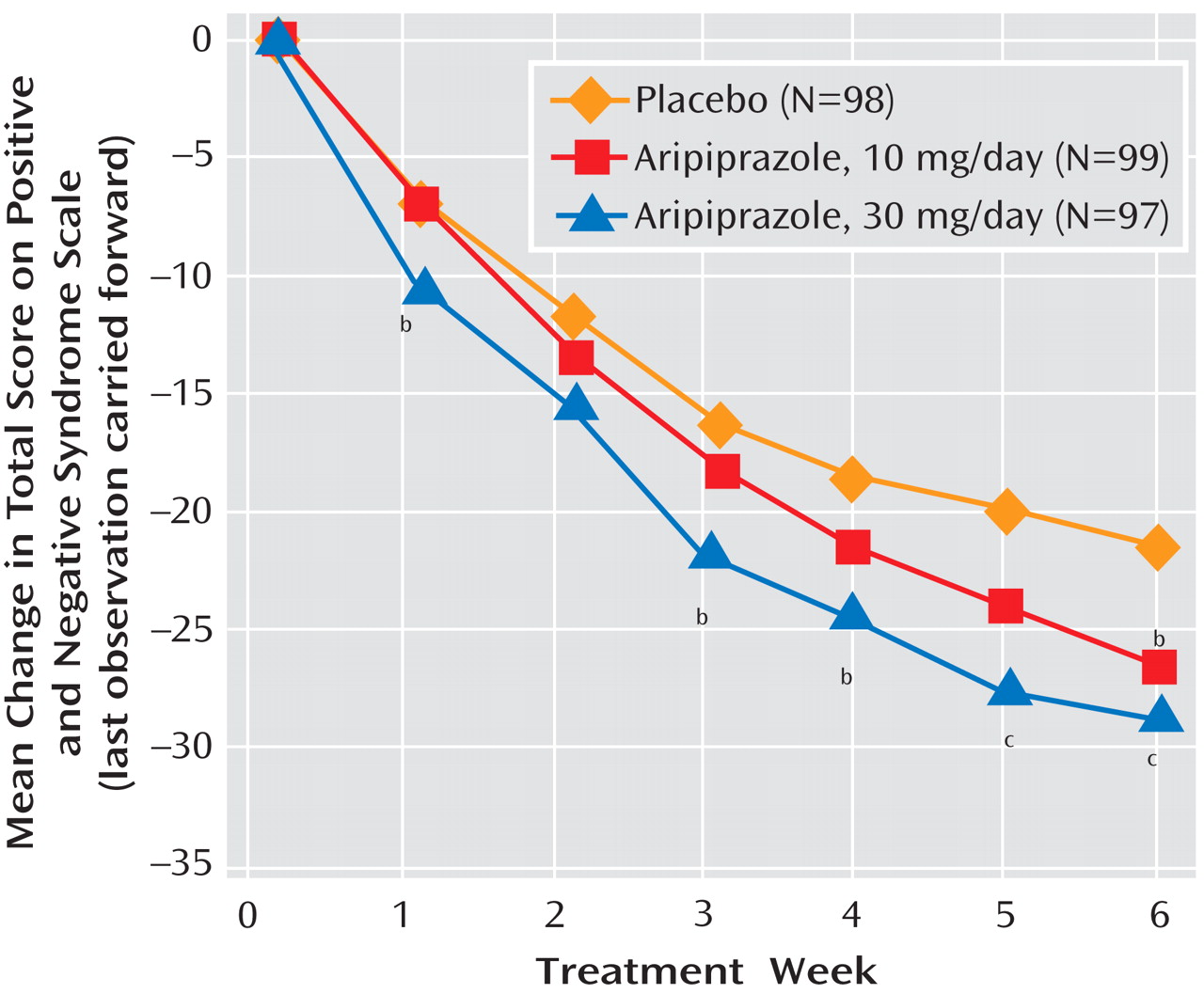
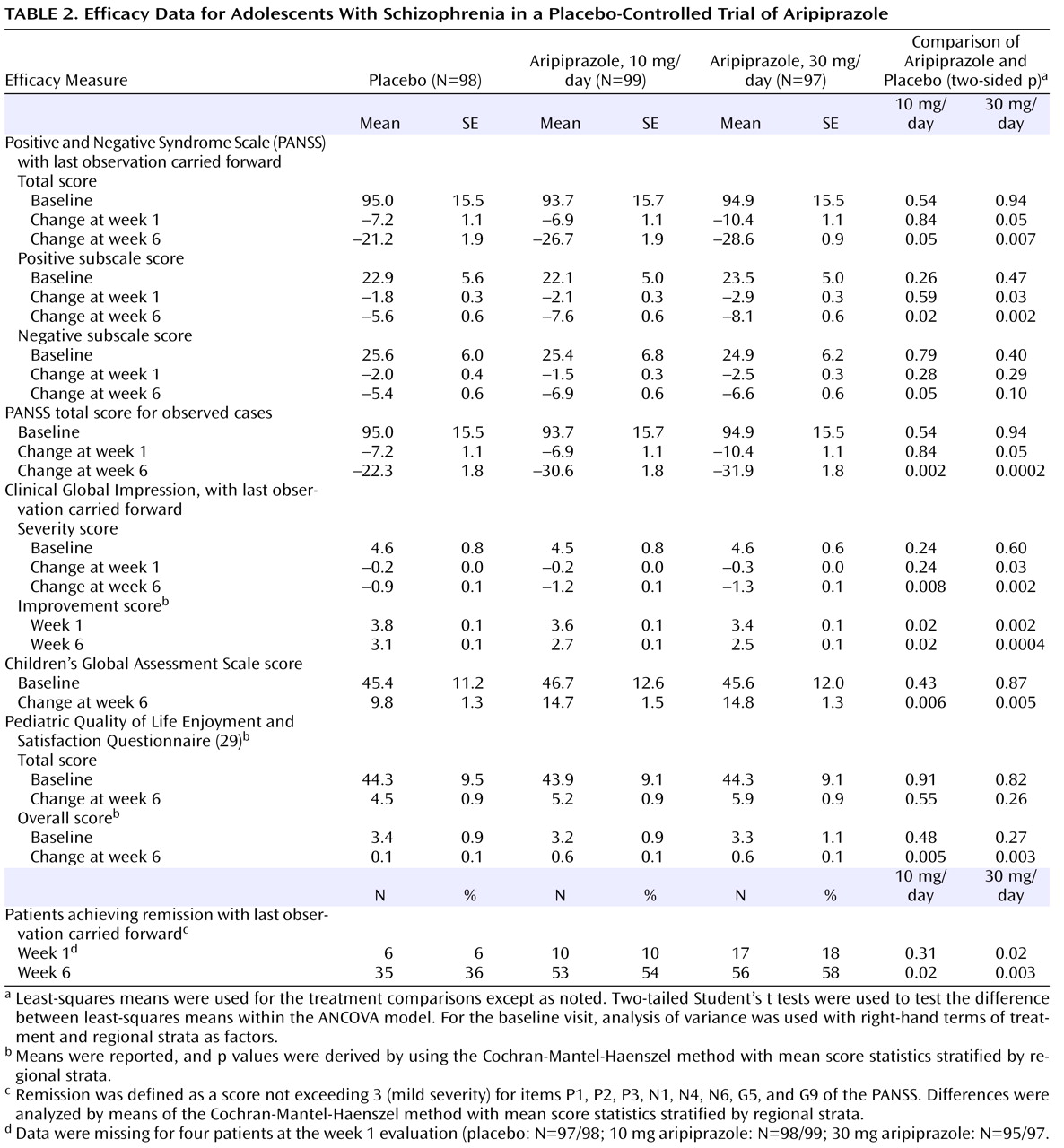
To our knowledge, this study with aripiprazole is the largest investigation conducted to date in adolescents with schizophrenia. Aripiprazole at doses of 10 and 30 mg/day was more efficacious in ameliorating the symptoms of schizophrenia than was placebo, as demonstrated by significantly greater improvements on the PANSS total score. Although considerable improvement was also observed with placebo, the salutary effects of placebo highlight the utility of employing a placebo-controlled methodology in treatment studies with this vulnerable adolescent population.
Statistically significant superiority for both active treatment arms was also demonstrated on the PANSS positive subscale scores at week 6. In addition, decreases were seen in PANSS negative subscale scores in both aripiprazole dosage groups. However, a statistically significant difference from placebo was observed in the 10-mg aripiprazole arm at study endpoint only. The 30-mg aripiprazole dose was superior to placebo at weeks 3 and 4 but not at endpoint (week 6 or last observation). End-of-study scores for the CGI severity and improvement scales demonstrated improvement consistent with a clinically meaningful response, with both dose groups showing significant reductions over the course of the study. Subjects with a “moderately” to “markedly” severe baseline illness (mean CGI severity score, 4.6) demonstrated clinically meaningful improvement, as shown by the CGI improvement scale. Both dose groups demonstrated statistically significant improvement compared to placebo, and the difference from placebo was numerically greater for the 30-mg group than for the 10-mg group at all scheduled postbaseline visits except week 2. Finally, after 6 weeks of treatment at either dose, scores on the Children’s Global Assessment Scale improved by more than 30% in both aripiprazole groups, compared with 22% in the placebo group.
The rate of discontinuation due to adverse events was low in both aripiprazole groups and was comparable to the rate in the placebo group. One patient in the 10-mg aripiprazole group withdrew owing to the emergence of dystonia, which resolved 3 days later
(26) .
While neither active treatment group exhibited substantial weight gain in the current study, the change in weight differed across groups, because of weight loss in the placebo group but not in the two active treatment groups. Thus, like the data for other antipsychotic medications, findings from the current trial suggest that clinicians treating adolescents should remained concerned about the potential for adverse long-term changes in weight with aripiprazole.
Hyperprolactinemia also has long been reported with antipsychotic therapy. The effects of hyperprolactinemia—anovulation, amenorrhea, decreased libido, orgasmic dysfunction, breast engorgement, galactorrhea, and hypoestrogenism/androgenism—can be disruptive and may be more pronounced in postpubertal adolescents
(35) . All treatment groups demonstrated a reduction from baseline in serum prolactin concentrations. Significant differences between placebo and aripiprazole may be related to the dopamine partial agonist properties of aripiprazole, which is thought to mimic the inhibitory action of dopamine on pituitary prolactin secretion. Few published data exist on the consequences of lowered prolactin in adult or in child and adolescent populations; however, possible consequences include failure to lactate in childbearing girls and possible decrease in pubic hair. In cases of extremely low prolactin (e.g., less than 2 ng/ml), there is the possibility of impaired fertility
(36 –
39) .
Despite the observation that overall clinical improvement has been demonstrated in adolescents with schizophrenia who receive placebo, and the lack of approved treatments for adolescent schizophrenia at the time this study began, ethical concerns about the use of placebo in clinical studies remain. In an attempt to reduce the risk associated with participation in this clinical trial, subjects were allowed to receive “rescue medication” (benzodiazepines and anticholinergics) over the course of the study. In addition, subjects who received placebo were given the option to receive aripiprazole at the end of the study. Furthermore, as a precautionary measure, a data safety monitoring board reviewed and evaluated cumulative safety data collected at regular intervals to ensure the safety of all subjects enrolled in this study.
The procedures used in this study addressed the methodologic limitations of most other studies of pharmacologic therapy in adolescent schizophrenia. Nevertheless, because schizophrenia generally requires long-term treatment, additional large, randomized, placebo-controlled clinical trials are needed to assess long-term outcomes. In addition, the study did not include patients with schizoaffective disorder; thus, caution should be used in applying these study results to that patient group. Finally, large-scale, randomized, head-to-head trials of typical versus atypical antipsychotics in adolescents are necessary so that between-drug comparisons can be made on the basis of methodologically stringent data.
In conclusion, aripiprazole was generally well tolerated, with a low rate of discontinuation due to adverse events and a high completion rate in adolescents with schizophrenia. These results provide support for aripiprazole use in adolescents with schizophrenia at a starting dose of 2 mg/day, and they provide evidence of efficacy and tolerability at the recommended adult doses, ranging from 10 to 30 mg/day. Longer-term, randomized, head-to-head trials of typical versus atypical antipsychotics are necessary to confirm the efficacy and safety of long-term management of schizophrenia in adolescents.