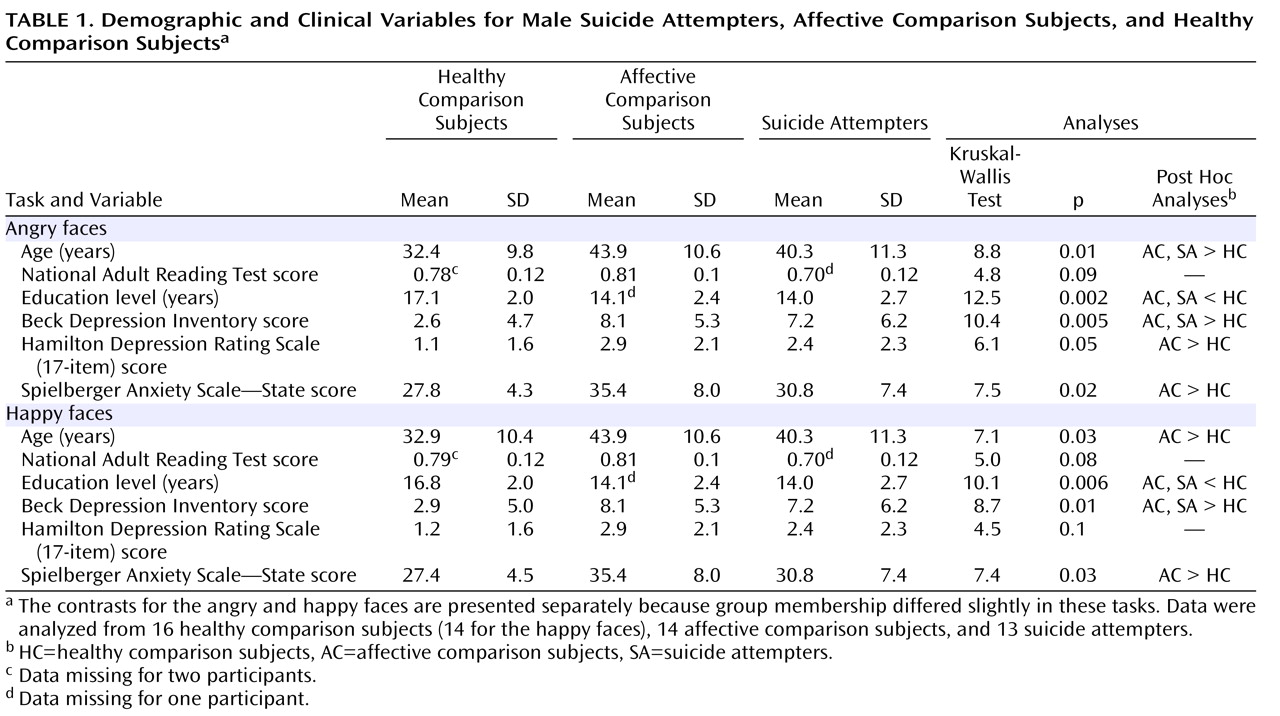
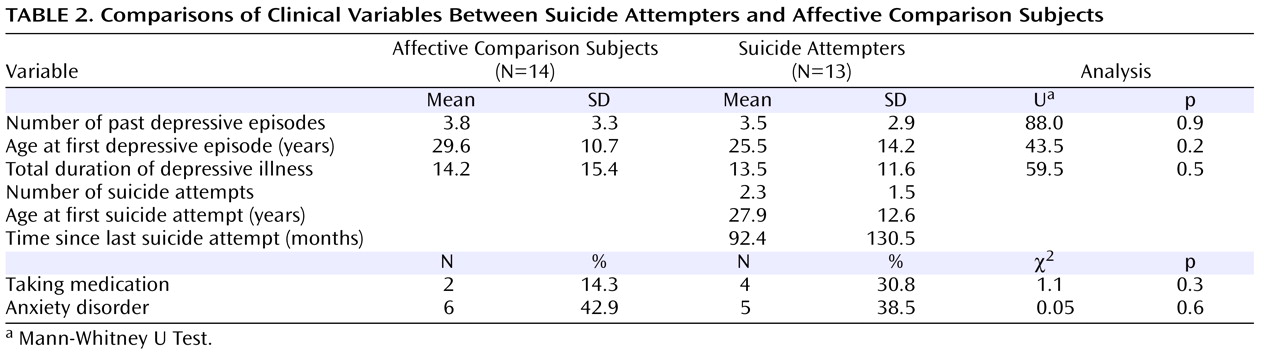
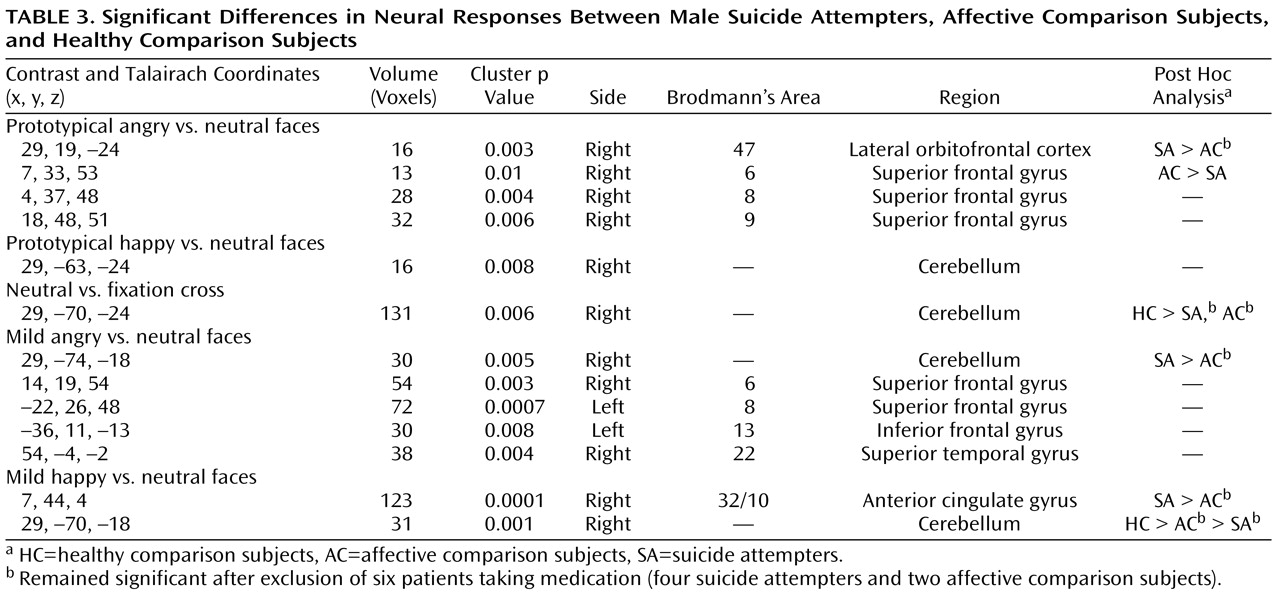
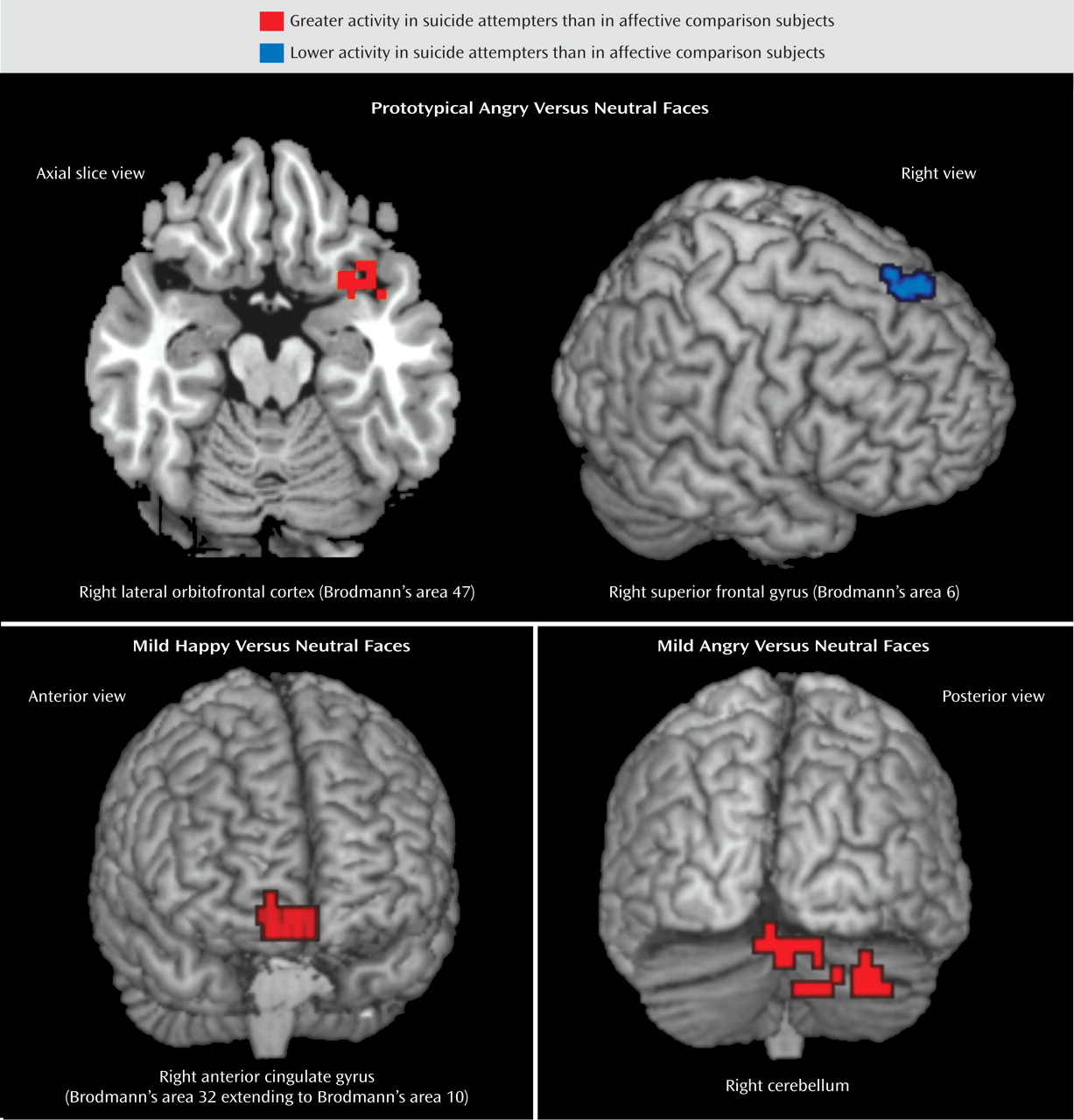
Considerable evidence links vulnerability to suicidal behavior with specific neurobiological abnormalities
(1) . These include abnormal serotonin receptor expression in the ventral prefrontal cortex in suicide completers
(2) and impaired performance on cognitive tasks that are dependent on this brain region in suicide attempters
(3,
4) . It has therefore been hypothesized that impaired serotonergic modulation of the ventral prefrontal cortex places individuals at risk for suicide after exposure to stressors that impair decision-making capacity in negative emotional contexts
(1,
4) .
Few neuroimaging studies have been conducted in relation to suicidal behavior. Recently Monkul and colleagues
(5) found that the right amygdala was smaller in female suicide attempters than in depressed comparison subjects and that the orbitofrontal cortex was smaller than in healthy comparison subjects. In a positron emission tomography (PET) study, Audenaert and colleagues
(6) found reduced 5-HT
2A receptor binding in the prefrontal cortex in depressed suicide attempters relative to healthy comparison subjects. Cannon and colleagues
(7) found decreased serotonin transporter binding in the midbrain and increased binding in the anterior cingulate gyrus in depressed bipolar patients with a history of suicidal acts compared with bipolar patients with no suicidal history. In another PET study, Oquendo and colleagues
(8) reported a lower glucose uptake in several regions of the prefrontal cortex (Brodmann’s areas 6, 8, 9, and 44) and the anterior cingulate gyrus (areas 24 and 32) in high- versus low-lethality suicide attempters. These studies link suicidal behavior with the serotonergic system and the prefrontal cortex, including the orbitofrontal, dorsolateral, and anterior cingulate cortices.
No study has specifically examined activity in neural systems engaged by negative emotional cues that are potentially relevant to suicidal behavior, such as social signals of disapproval. In suicide attempters, hypersensitivity to social signals of disapproval may engender extreme feelings of “thwarted love” and “avoidance of shame and humiliation” (
9, p. 25). Examining the neural response to such social signals may elucidate brain regions where suicide attempters exhibit perturbed responses to negative socioemotional cues.
To examine the neural correlates of suicidal behavior, we compared neural activity in male patients with a history of major depressive disorder either with or without a history of suicidal acts (“suicide attempters” and “affective comparison subjects,” respectively). Our study design called for patient groups with major depression because of the high attributable risk for suicidal acts associated with this diagnosis
(10) . To exclude any potential effect of depressed mood per se on abnormal neural activity, our study required that participants be euthymic at the time of scanning. We employed a well-validated event-related functional MRI (fMRI) task previously used to map neural responses to emotionally valent social cues in depression. This task involves presentation of modified Ekman and Friesen facial expression stimuli
(11,
12), using emotional expressions relevant to the study of suicidal behavior: observer-directed disapproval expressions (angry faces) and positive social expressions (happy faces) versus neutral faces. Our study therefore maps significant differences in neural activity to emotional versus neutral expressions between suicide attempters and affective comparison subjects. On the basis of previous findings, we hypothesized that 1) suicide attempters relative to affective comparison subjects would show perturbed function in the orbitofrontal, dorsal prefrontal, and anterior cingulate cortices; and 2) this perturbation would be present in response to angry versus neutral facial expressions, but not to happy versus neutral facial expressions or to neutral faces per se.
We also included a sample of healthy comparison subjects to determine the degree to which any differences between the patient groups represent deviations from health as opposed to mere differences between two pathological groups, neither of which differs from healthy comparison subjects. Moreover, the observation of perturbed neural activity in both patient groups relative to healthy comparison subjects provides insights on brain regions linked to major depressive disorder per se, independent of any association with past suicidal behavior.
Method
Participants
Participants were recruited through newspaper advertisements. They were first screened for inclusion criteria by telephone and then in person by an experienced psychiatrist. All participants were right-handed
(13) and male. Only men were included in this study because another paradigm studied within the same experimental session had previously shown gender effects. Three groups of participants were included: suicide attempters—individuals with a past history of both major depressive disorder and suicidal behavior; affective comparison subjects—individuals with a past history of major depressive disorder but no history of suicidal acts; and healthy comparison subjects—individuals with no past history of any DSM-IV axis I diagnosis.
Suicidal behavior was defined as any act carried out with the intent to die
(14) . Diagnoses were made according to DSM-IV criteria using the Mini-International Neuropsychiatric Interview, version 5.0.0
(15) . All participants had to be euthymic at the time of scanning, as indicated by a Hamilton Depression Rating Scale score <7
(16) . Other exclusion criteria included a lifetime history of severe head trauma, CNS disorder, bipolar disorder, schizophrenia, and a history of alcohol or drug abuse or dependence within the past 12 months.
Several additional assessments were conducted prior to scanning. We administered the English version of the National Adult Reading Test
(17) to provide an estimate of premorbid verbal IQ; the Beck Depression Inventory
(18) for a subjective measure of depressive state; and the Spielberger Anxiety Scale—State
(19) for current level of anxiety. The most recent and the most severe suicidal acts were assessed with the Risk Rescue Rating Scale
(20) and the Suicide Intent Scale
(21) . Finally, for data on relevant personality traits, we assessed impulsivity with the Barratt Impulsiveness Scale
(22), affective lability with the Affective Lability Scale
(23), anger with the State-Trait Anger Expression Inventory
(24), and trait anxiety with the Spielberger Anxiety Scale—Trait
(19) .
Forty-eight participants were examined. Two healthy individuals were unable to remain in the scanner because of anxiety. For technical reasons, data from two healthy comparison subjects for the angry faces task could not be analyzed, as well as data from two other healthy comparison subjects for the happy faces task. One suicide attempter was excluded because of current depression. Thus, data from 13 suicide attempters, 14 affective comparison subjects, and 16 healthy comparison subjects (14 for the happy faces) were analyzed.
Two of the affective comparison subjects and three of the suicide attempters were each taking one antidepressant, and one suicide attempter was taking one antidepressant and lithium at the time of scanning. No other participants were taking psychotropic medications.
The Ethics Committee (Research) of the Maudsley Hospital and Institute of Psychiatry approved the study protocol, and all participants provided written informed consent before entering the study. Study subjects were paid £30 for their participation in the study.
Stimulus Presentation
Each participant completed two consecutive 6-minute scanning runs. One run presented faces expressing happiness, and the other presented faces expressing anger from standardized series of prototypical faces posed by 10 different individuals
(11,
25,
26) . The order of runs was counterbalanced across individuals and groups. Each run included 20 pictures of one emotion at mild (50%) intensity, 20 pictures of the same emotion at prototypical (100%) intensity, and 20 neutral faces in a randomized order.
The mild emotion expressions were included because they represent facial expressions more frequently seen in everyday life and may be particularly relevant for the study of psychiatric disorders characterized by impaired interpersonal functioning, including mood disorders
(27,
28) . These stimuli were used in exploratory analyses rather than for exploring our main study hypotheses, which focused on the contrast between prototypical emotional and neutral faces.
The interstimulus interval varied from 3 to 8 seconds (mean=4.9 seconds) according to a Poisson distribution to prevent participants from predicting the timing of the next stimulus presentation. During the interstimulus interval, participants viewed a fixation cross (baseline).
Participants viewed each face for 2 seconds and were asked to identify the gender of each face and to press one of two buttons accordingly with the right hand (thus, this was a covert emotion processing paradigm).
Image Acquisition
Magnetic resonance images were acquired with a GE Signa 1.5-T Neuro-optimized MR system (General Electric, Milwaukee) at the Maudsley Hospital, London. An inversion recovery echo-planar imaging (EPI) data set was acquired at 43 near-axial 3-mm-thick planes parallel to the anterior-posterior commissure line (time to echo=40 msec, time to inversion=180 msec, time to recovery=16 seconds, in-plane resolution=1.72 mm, interslice gap=0.3 mm, and matrix size=128 × 128 pixels). This higher-resolution EPI data set provided whole brain coverage and was later used to register the fMRI data sets acquired from each individual in standard stereotactic space. A total of 180 T 2 -weighted images depicting blood-oxygen-level-dependent contrast were acquired at each of 25 near-axial noncontiguous 5-mm-thick planes parallel to the intercommissural line (time to echo=40 msec, repetition time=2 seconds, in-plane resolution=3.44 mm, interslice gap=0.5 mm, and matrix size=64×64 pixels).
fMRI Data Analysis
Data were analyzed using the XBAM software package, version 3.4
(29), developed at the Institute of Psychiatry (see www.brainmap.co.uk for more information). XBAM is based on a nonparametric approach. Analysis of covariance was carried out on the effect size maps in Talairach and Tournoux’s standard space
(30) . We included in the analysis all those voxels where at least half the participants had data present. Each voxel statistic was then corrected for the actual number of participants contributing to the calculation
(31) .
To directly test our main hypotheses, we employed a one-way analysis of covariance (ANCOVA) examining the main effect of diagnostic group (three levels) on whole-brain neural activity to prototypical angry versus neutral expressions and to prototypical happy versus neutral expressions. These ANCOVAs were followed by post hoc whole-brain pairwise contrasts between groups, and only clusters that were significant at the main ANCOVA level and the pairwise level are reported. Age and level of education were used as covariates in comparisons between healthy comparison subjects and the patient groups. In a second step, pairwise between-group analyses were conducted after exclusion of medicated patients. A clusterwise p value of 0.01 (corrected for whole brain volume using permutation testing) was used for all analyses.
Assessment of Explicit Emotion Identification
After each scanning session, all participants underwent two tests to assess their ability to process facial information. The Short Recognition Memory Test for Faces
(32) provided an indirect measure of nonemotional face perception, and a computerized test of facial emotion recognition was conducted (see reference
28 for details).
Statistical Analyses
Statistical analyses other than the fMRI analyses were carried out with SPSS (SPSS, Inc., Chicago). Because of the small size of the groups, only nonparametric tests were used. Comparisons of quantitative data between the three groups were made by means of Kruskal-Wallis tests and between pairs of groups by Mann-Whitney U tests. Associations between qualitative variables and groups were calculated with chi-square tests. Spearman correlations were used for correlation analyses. The Bonferroni correction was applied when multiple correlations were conducted. The alpha level was set at 0.05.
Results
Demographic and Clinical Comparisons
Tables 1 and
2 summarize demographic and clinical details and statistical comparisons. Suicide attempters did not differ from affective comparison subjects in any of the clinical variables considered.
There were no significant differences in personality traits between the patient groups after the Bonferroni correction was made.
Main Effect of Group for Prototypical Facial Expressions
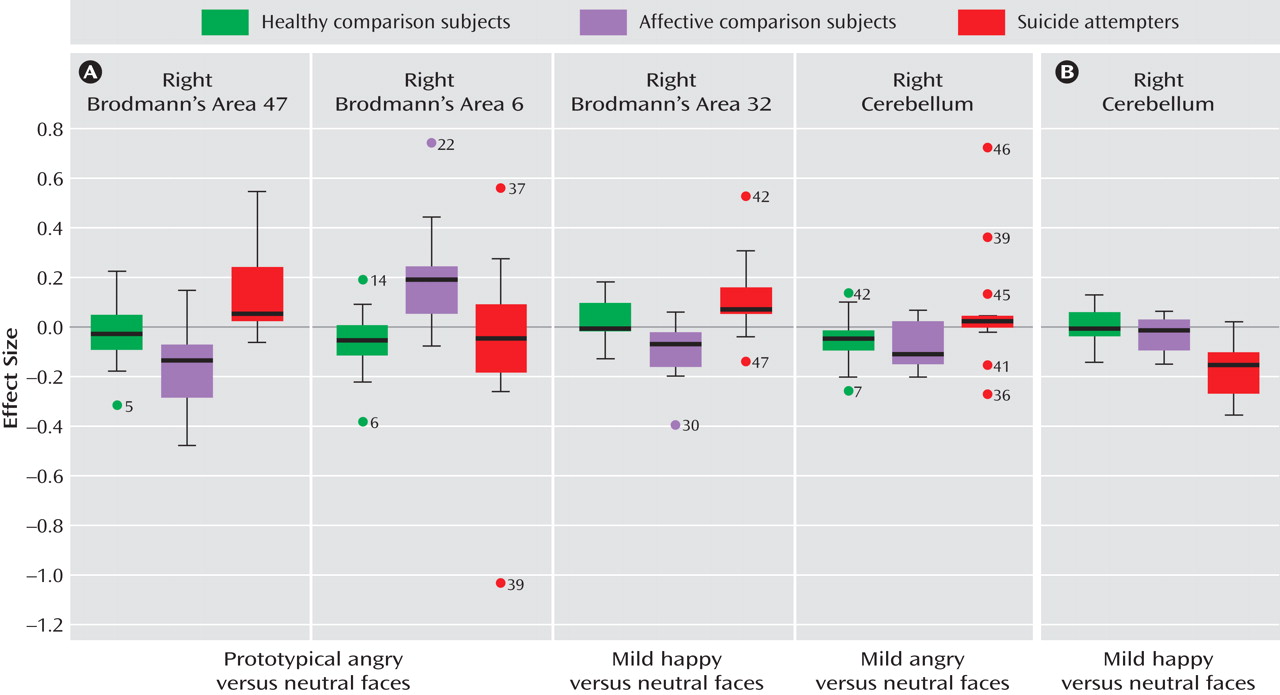
We examined the effect of group and interaction with facial expression on neural activity in response to each emotion at full intensity versus neutral faces to test our main hypotheses and to control for any between-group difference in neural activity to faces per se (i.e., neutral faces). This analysis revealed a significant interaction of group by prototypical (happy and angry) facial expression (3×2) in the right anterior cingulate (Brodmann’s area 24/32) and the left superior frontal gyrus (Brodmann’s area 8). Comparison of effect size values revealed significant differences in activity in both regions between prototypical angry and happy conditions for healthy comparison subjects, but not for either patient group.
We then conducted a one-way analysis of variance of the contrast of angry versus neutral faces and happy versus neutral faces with group as the between-subjects factor. To prototypical anger versus neutral facial expressions, there was a significant main effect of group in the posterior part of the right lateral orbitofrontal cortex (Brodmann’s area 47) and in three regions of the right superior frontal gyrus (Brodmann’s areas 6, 8, and 9). Post hoc whole-brain pairwise between-group analyses were then conducted. Suicide attempters showed significantly greater activity than affective comparison subjects in the right lateral orbitofrontal cortex (Brodmann’s area 47), and affective comparison subjects showed significantly greater activity than suicide attempters in the right superior frontal gyrus (Brodmann’s area 6). When patients receiving medication were excluded from these pairwise between-group analyses, the right Brodmann’s area 47, but not area 6, remained significant for this comparison. There were no significant differences in neural activity to prototypical angry versus neutral facial expressions between healthy comparison subjects and either patient group.
To prototypical happy versus neutral facial expressions, the main effect of group was significant only in the right cerebellum. Post hoc whole-brain between-group comparisons yielded no statistically significant differences between any group pairs, however.
Main Effect of Group for Neutral Facial Expressions
To exclude a potential effect of responses to neutral facial expression in these findings, we examined the effect of group on neural activity to neutral facial expressions versus the fixation-cross baseline. There was a significant effect of group only in the right cerebellum. Post hoc pairwise between-group analyses revealed significantly greater activity in the right cerebellum in healthy comparison subjects than in both patient groups, but not between patient groups. These differences remained significant after exclusion of medicated patients.
Main Effect of Group for Mild Facial Expressions
We conducted exploratory analyses to examine between-group differences in activity in response to the viewing of mild emotional versus neutral facial expressions. To mild anger versus neutral facial expressions, there was a significant main effect of group in the right cerebellum, the left inferior frontal gyrus (Brodmann’s area 13), the right superior frontal gyrus (area 22), the left superior frontal gyrus (area 8), and the right superior frontal gyrus (area 6). Post hoc whole-brain pairwise analyses revealed significantly greater activity in the right cerebellum in suicide attempters compared with affective comparison subjects. This finding remained significant after exclusion of medicated patients. There were no significant between-group differences in neural activity in either of the other pairwise comparisons.
To mild happy versus neutral facial expressions, there was a significant main effect of group in the right cerebellum and a large cluster extending from the right anterior cingulate gyrus (Brodmann’s area 32) to the medial frontal gyrus (area 10). Post hoc whole-brain pairwise analyses revealed greater activity in the right cerebellum in healthy comparison subjects than in both patient groups, and in affective comparison subjects than in suicide attempters. Suicide attempters showed greater activity than affective comparison subjects in the right Brodmann’s area 32/10. These findings remained significant after exclusion of patients taking medication.
Gender Recognition During fMRI Sessions: Reaction Time and Accuracy
No significant group differences were observed in accuracy or reaction time during gender recognition for happy or angry facial expressions. These results indicate no between-group differences in attention to facial expressions during scanning.
Facial Processing Abilities
Significant between-group differences were observed in the nonemotional Short Recognition Memory Test for Faces. There were also no significant between-group differences in accuracy or reaction time for explicit emotion recognition after Bonferroni correction.
Discussion
As hypothesized, patients with a history of suicidal behavior showed different responses to prototypical angry faces relative to well-matched patients with no such history in several regions of the prefrontal cortex; we observed increased activity in the right lateral orbitofrontal cortex (Brodmann’s area 47) and decreased activity in the right superior frontal gyrus (area 6). These differences were not seen in response to neutral or prototypical happy faces. The increased activity in area 47 in suicide attempters in response to angry faces survived exclusion of six patients taking medication. Moreover, neither patient group differed from healthy subjects in the contrasts that differentiated previously depressed patients with and without histories of suicidal behavior. Contrary to our second hypothesis, there was also significantly greater activity in the right anterior cingulate gyrus (Brodmann’s area 32, extending to the medial frontal gyrus, area 10) in suicide attempters compared with affective comparison subjects in response to mild (but not prototypical) happy versus neutral faces. Finally, suicide attempters also showed significantly greater activity than affective comparison subjects in the right cerebellum in response to mild angry expressions. These regions have previously been implicated in suicidal behavior
(2,
5,
7,
8) .
Brodmann’s area 47 has been implicated in the processing of facial expressions
(33), particularly in the processing of anger
(34,
35) . Moreover, area 47 may be especially responsive to subjectively salient or mood-congruent expressions. For example, in a previous study
(36), depressed patients showed greater activity than healthy individuals in the right Brodmann’s area 47 in response to mood-congruent sad versus mood-incongruent happy covert stimuli. Our findings therefore suggest that angry faces may be an especially salient stimulus for individuals at risk for suicide in the same way that sad signals are salient for depressed individuals. Angry faces are a social signal of disapproval indicating that one’s current behavior is unacceptable and should be modified. Regions of area 47 (in the lateral orbitofrontal cortex) are also implicated in the receipt of punishment
(37) . Suicide attempters may display increased sensitivity to others’ disapproval and greater perception of punishment in these contexts. Notably, however, our study failed to detect differences in area 47 function in either patient group relative to healthy comparison subjects. This contrasts with prior findings differentiating area 47 function in depressed and healthy subjects. This difference between findings in this study and prior studies
(36) could be related either to the relatively small sample sizes we used here or to the fact that all patients in this study were examined while euthymic, whereas other studies have examined subjects during depressive episodes.
Suicide attempters showed significantly lower activity than affective comparison subjects in the right superior frontal gyrus (Brodmann’s area 6) in response to prototypical angry versus neutral faces, although this finding was no longer significant after exclusion of the patients taking medication. Area 6 has been implicated in control of voluntary actions in conflict situations
(38) . A PET study in suicide attempters
(8) reported a negative correlation between impulsivity and area 6 activity at rest. Lower activity in area 6 in suicide attempters may thus be linked to a higher propensity to act in response to negative emotions.
Suicide attempters also showed greater activity than affective comparison subjects in the right Brodmann’s area 32 extending to area 10 in response to mild but not prototypical happy faces. However, as with the finding for area 47, neither patient group differed from healthy comparison subjects. There may be greater ambiguity in mild emotional expressions, and mild emotional expressions presented with neutral expressions may be less easily distinguishable from each other. Regarding its role in attention to and effortful regulation of affective states
(39), greater activity in area 32 in suicide attempters may therefore reflect increased effortful attentional processing of ambiguous positive emotional stimuli. We hypothesize that suicidal patients may thus display more difficulty in finding protective (positive) factors in their environment.
Healthy individuals showed significantly greater activity than both patient groups in the right cerebellum in response to neutral faces and to mild happy expressions. These findings parallel our previous results
(27) in currently depressed patients compared with healthy individuals. Together, they provide replicable evidence of perturbed cerebellar function in individuals with either current or past depression, suggesting state-independent dysfunction. These findings add to the literature highlighting the role of the cerebellum as a key component of neural systems underlying emotion processing
(40) .
More research is needed on the neural differences between depressed patients facing low versus high risk for suicidal behavior. Continued research on the neural response to social cues may provide insights, given that suicidal behavior often occurs when vulnerable individuals enter negative socioemotional contexts. Our findings of neural responses to facial expressions, an important class of socioemotional cues, may provide important clues to understanding these differences. Comparisons of neural activity among the three groups (
Figure 2 ) showed that responses tended to be in opposite directions in suicide attempters and affective comparison subjects relative to healthy comparison subjects in regions associated with the vulnerability to suicidal behavior but in similar directions in regions associated with the vulnerability to depression. More research is needed to examine commonalities and differences in neural response to social signals among these three groups.
There are some limitations to our study. First, our findings may not generalize to all suicide attempters, as our population only included male, mostly middle-aged, individuals with a history of major depressive disorder. It will be important in the future to study different populations, such as women, adolescents, older age groups, and individuals with other psychiatric disorders associated with suicidal behavior. Second, the relatively small size of our groups may have limited our ability to detect further differences between groups, although our sample size remains comparable to those of previous neuroimaging studies of suicide attempters. Third, comparisons between patients and healthy individuals may have been blunted by the need to control for age and education. In future research, more careful matching of the three groups would enable better analyses (without covariates) and perhaps reveal a network of brain regions associated with vulnerability to depression. Fourth, we did not assess the duration of symptom-free periods in our patient groups. Finally, medication is a frequent potential confounding factor in functional imaging studies of patients, particularly when studying euthymic previously depressed individuals. In this study, few individuals were taking medication at the time of scanning. Even after exclusion of these patients in secondary analyses, however, most between-group differences in neural activity remained significant.