Studies using functional MRI (fMRI) in young depressed patients have reported activity changes in the limbic-thalamic-cortical network associated with emotion and cognition
(1 –
5) . Several studies have described exaggerated activity in the ventral frontal and limbic regions, including the amygdala
(2,
4,
6), in young adults with major depression relative to comparison subjects during negative emotional processing, although this activity is not always found
(7) . Depending on the degree of task difficulty, depressed patients also show increased or decreased activation in the dorsolateral prefrontal cortex while performing cognitive tasks
(5,
8 –
10) . Antidepressant medication normalizes these state-dependent changes in those patients who respond to treatment
(2,
6,
11 –
14) .
In contrast to studies of young depressed adults, fMRI studies that evaluate both emotional and cognitive function in geriatric depression are scarce. Elderly patients with depression may show deficits different from those of young depressed adults, given age-related changes such as cerebrovascular ischemia and white-matter hyperintensities
(14) . A greater range of adverse outcomes, including cognitive impairment, has been reported in late-onset depression. This may reflect the greater presence of cerebrovascular pathology in geriatric depression, particularly in the frontostriatal regions, leading to cognitive and emotional deficits
(15) . Aizenstein et al.
(16) demonstrated decreased activation in the dorsolateral prefrontal cortex and increased activation in the striatum in depressed older patients relative to comparison subjects during an explicit sequence learning task. However, their study did not investigate the relationship between altered brain activation and clinical status. There has been no comparable study investigating the emotional processing system in geriatric depression. It is likely that abnormal activation in the frontostriatal circuits during a cognitive task is a prominent feature of geriatric depression rather than exaggerated activation in emotion-related regions during a negative emotional task.
While there have been many neuroimaging studies of young patients during a depressive state, only a few studies of remitted patients have been reported. Decreased activity of the medial and orbital frontal cortices in remitted patients during sad mood induction has been reported, indicating a possible neuroimaging marker of depression
(17,
18) . This possibility has not been studied in geriatric depression. Decreased activation in the dorsal anterior cingulate cortex during a verbal fluency task has been observed in remitted patients who had multiple depressive episodes
(19) . However, the study did not include an acutely depressed group for comparison. A systematic comparison of acutely depressed patients, patients with remitted depression, and normal healthy subjects is needed to identify the depressive state-related and disease-related alterations in brain activities in geriatric depression.
In addition to activation, task-induced negative activation
(20) —or deactivation—might also have implications in geriatric depression. Greicius et al.
(21) recently reported greater resting-state subgenual cingulate and thalamic functional connectivity in young depressed patients relative to healthy comparison subjects. Regions in the posterior cingulate revealed a reduced magnitude of deactivation in mild cognitive impairment and Alzheimer’s disease
(22,
23) . These studies highlight the importance of investigating the association of deactivation in the default-mode network with depressive state and cognition.
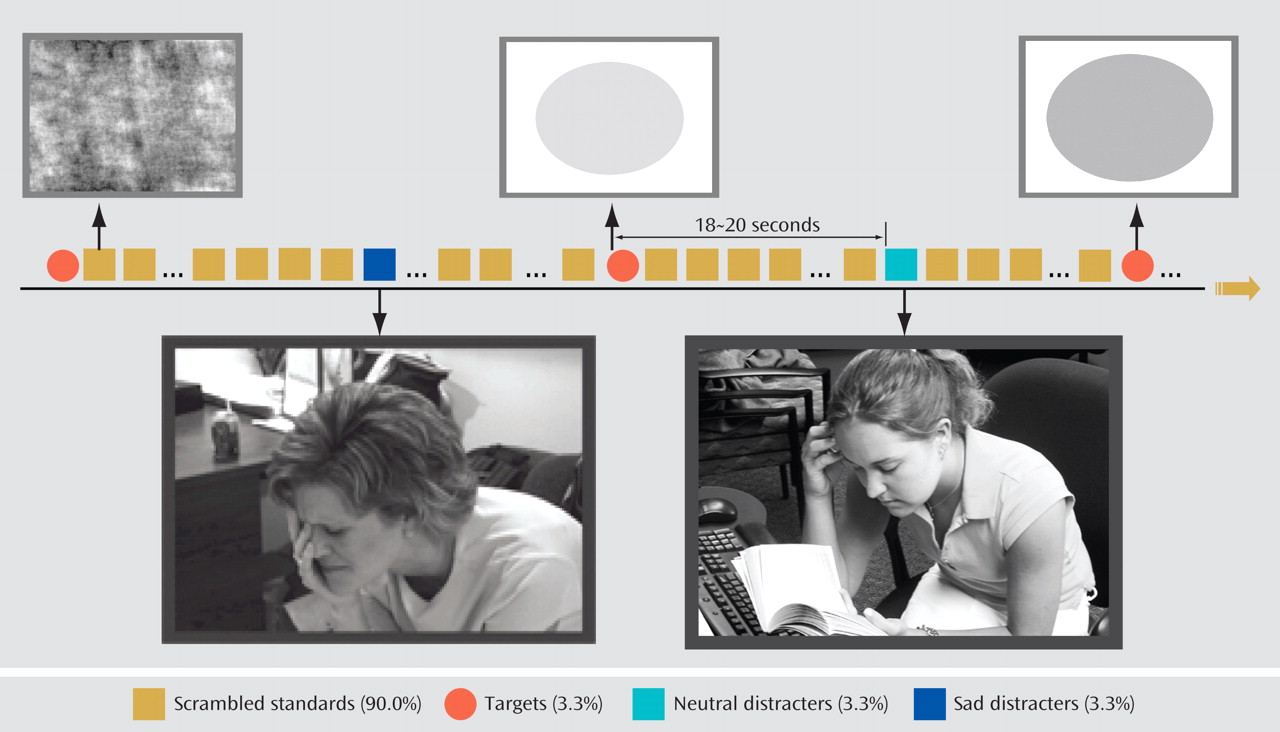
In this study, we compared brain activity in acutely depressed patients, patients with remitted depression, and healthy comparison subjects, all over age 60. We used an emotional oddball task during fMRI scanning to evaluate both emotional and executive processing. Given that apathy and cognitive dysfunction might be prominent in geriatric depression, we hypothesized that relative to the comparison subjects, acutely depressed patients would exhibit decreased activation in the executive system to attentional targets. On the other hand, we hypothesized that they would not show hyperactivity in the emotional system in response to sad stimuli. We also surmised that some of these features might constitute a disease-specific biomarker and would be present in patients whose depression had remitted. Finally, we hypothesized that depressed patients would have enhanced deactivation because of limited attentional resources.
Discussion
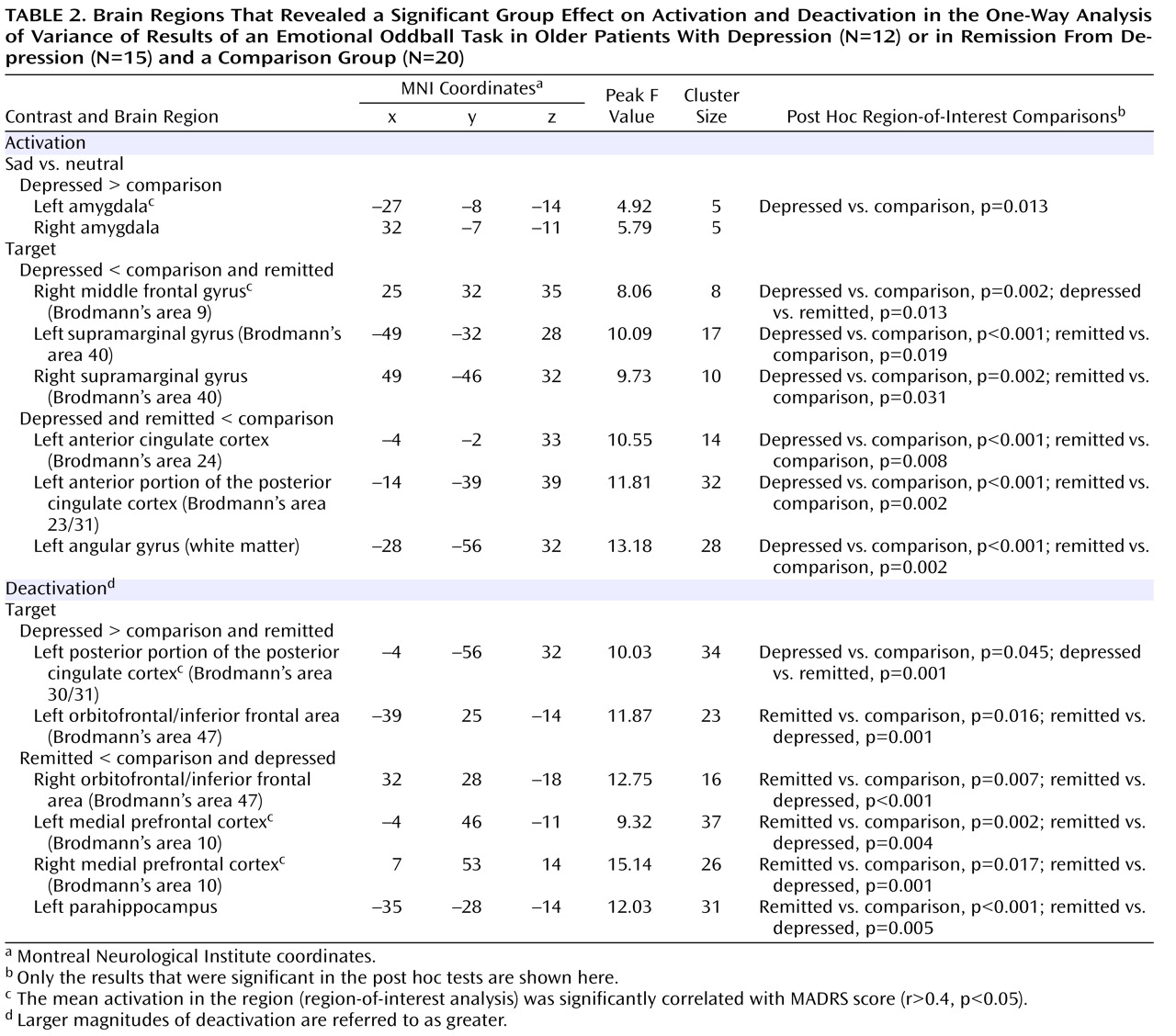
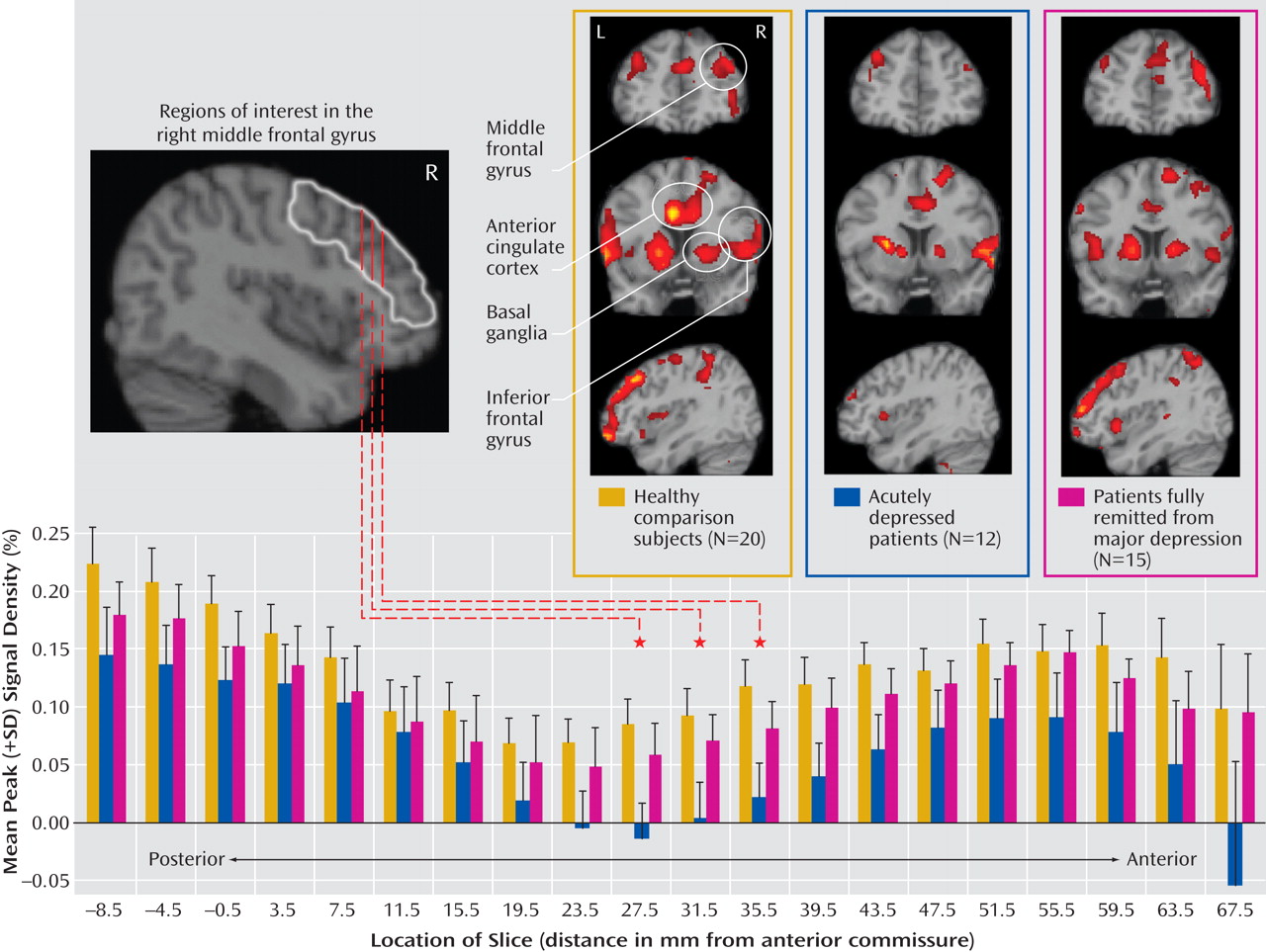
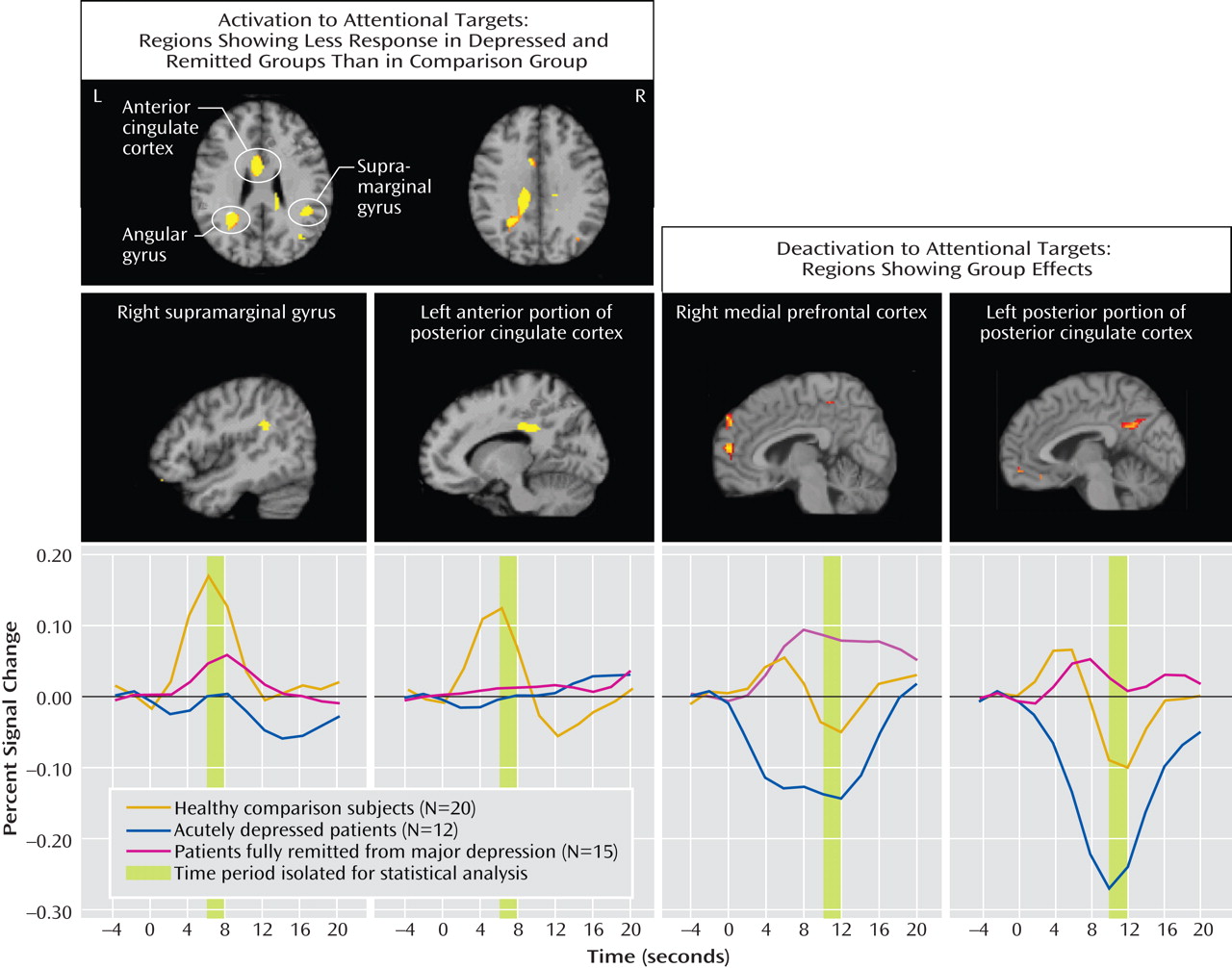
The major findings of this study of older individuals were several. First, acutely depressed patients showed decreased activation in the right middle frontal gyrus during target detection, which was depressive state dependent. Second, remitted patients demonstrated attenuated activation during target detection comparable to that of the depressed patients in the cingulate and inferior parietal areas, suggesting disease-related alterations in these regions. Third, while the attenuated activation of the anterior portion of the posterior cingulate might be disease related, the enhanced deactivation of the posterior portion was depressive state dependent, which suggests that the anterior and posterior portions of the posterior cingulate appear to have distinct roles in geriatric depression. Finally, the remitted group had diminished deactivation in the default-mode network during target detection. Notably, these significant differences among the three groups were mainly revealed during target detection but not during emotional processing, which suggests that executive dysfunction is a prominent deficit in geriatric depression. The changes in response to emotional stimuli in geriatric depression will need further confirmation in larger samples.
The attenuated activation to targets in the depressed group in the middle frontal gyrus (dorsolateral prefrontal cortex) is consistent with findings from Aizenstein et al.
(16) and with our findings in young depressed patients using the same task
(30) . The significantly increased activation in this region in the remitted group relative to the acutely depressed group is also consistent with some of the studies of young depressed patients
(2,
3,
5,
31,
32), which showed normalized activation after medication. Thus, our results confirm the important contribution of the right dorsolateral prefrontal cortex to the depressive state.
The inferior parietal and cingulate regions have been consistently activated during attentional and working memory tasks
(26,
28,
33) . Alterations in these regions in both patient groups indicate executive dysfunction, which could be related to factors that are primarily associated with pathological changes in geriatric depression, such as those caused by cerebrovascular disease. Although a causal link between ischemic lesions and geriatric depression remains controversial
(34), ischemic lesions, particularly white matter hyperintensities, may explain in part the persistently attenuated activation in fully remitted depressed patients. Interestingly, persistent attenuation was also observed in white matter areas (the superior longitudinal fasciculi), which has been related to an indirect effect of white matter hyperintensities. Alternatively, the alteration could be a disease marker reflecting a “scar” left from prior depressive episodes, even in the absence of clinical symptoms. Further evidence of association between fMRI findings, diffusion tensor imaging findings
(35), and the residual symptoms in remitted patients would help to elucidate the significance of the persistently attenuated activation in these regions.
The anterior portion of the posterior cingulate (Brodmann’s area 23/31) is heavily interconnected with the dorsal anterior cingulate cortex (Brodmann’s area 24) and the dorsolateral prefrontal cortex (Brodmann’s area 46)
(36) and appears to be one of the key nodes of the attention-cognition component in Mayberg’s depressive model
(37) . Our data suggest a strong link of this anterior portion of the posterior cingulate region with the pathology of geriatric depression. The posterior portion (Brodmann’s area 30/31) is more closely connected with the retrosplenial cortex and the hippocampal complex. Together with the precuneus, the region of the posterior portion of the posterior cingulate has been implicated in self-consciousness and memory retrieval
(22) . The enhanced deactivation of this region in the acutely depressed patients, which is opposite to the changes in mild cognitive impairment and Alzheimer’s disease
(22,
23), implies that it may be possible to differentiate between cognitive dysfunction in acute depression and cognitive changes seen in mild cognitive impairment. Lack of deactivation in Alzheimer’s disease and mild cognitive impairment might suggest less task engagement and impaired memory retrieval processing, whereas the increased deactivation in acutely depressed patients might reflect increased task processing demands due to limited attentional resources or baseline hyperactivity due to an active tendency toward rumination.
The lack of deactivation in our remitted group might reflect a biomarker in this group that might signify the intermediate state, characteristic changes related to antidepressants or depressive episodes, cognitive decline, or a response secondary to vascular change. Our remitted group tended to have more depressive episodes and a longer period of exposure to antidepressants than the acutely depressed group. More depressive episodes tended to be correlated with less deactivation of the posterior portion of the posterior cingulate in the remitted group but not in the acutely depressed group, which suggests a stronger influential effect of remission state than of number of depressive episodes. A preliminary comparison between the six acutely depressed and six remitted patients who were on monotherapy with an SSRI (data not shown) revealed that the differences in activations and deactivations between the two groups remained significant (two-sample t test, p<0.01). Thus, the state of depression or remission seemed to have a greater effect on our results than use of antidepressants. However, our sample is too small to support this conclusion. The effect of antidepressant medication on vigilance, arousal, and attention varies greatly among the antidepressants
(38), and the influence of different antidepressants on deactivation associated with vigilance, arousal, or attention remains to be clarified. The Mini-Mental State Examination score in the remitted group was relatively higher than in the acutely depressed group. Therefore, it is also less likely that the diminished deactivation was due to more severe cognitive dysfunction in the remitted group. In short, the lack of deactivation appeared to be a characteristic change related to the remission state. Nevertheless, further studies with antidepressant medication-free patients, studies of the effects of different antidepressants, and detailed evaluation of cognitive function among the three groups will be important for understanding the deactivation pattern in the remitted subjects.
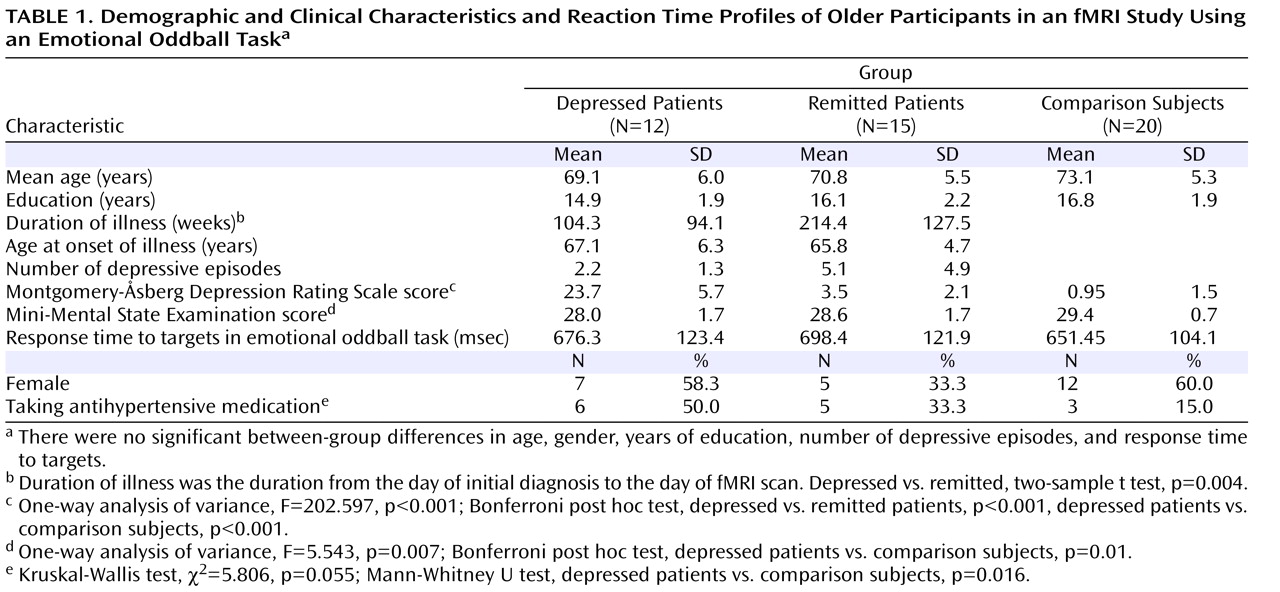
In addition to antidepressants, antihypertensive medications could affect participants’ hemodynamic response. The ratio of participants with to those without antihypertensive medication in the depressed group was higher than in the remitted and comparison groups (
Table 1 ). In future studies, matching hypertension and possibly other medical conditions as well as combining perfusion images in larger samples should be undertaken.
To our knowledge, this is the first fMRI study to evaluate emotional and cognitive function simultaneously in geriatric samples of patients with depression and patients remitted from depression. The attenuated activation in the parietal and cingulate regions, including in white matter areas, in both the depressed and remitted groups seem to be pathology related and warrant further investigation. Notably, this study suggests an important role for the posterior cingulate cortex in geriatric depression. Future studies directly comparing task-induced deactivation in patients with depression and mild cognitive impairment, patients with depression and no mild cognitive impairment, and patients with mild cognitive impairment and no depression may help in clinical differentiation and intervention in geriatric depression.