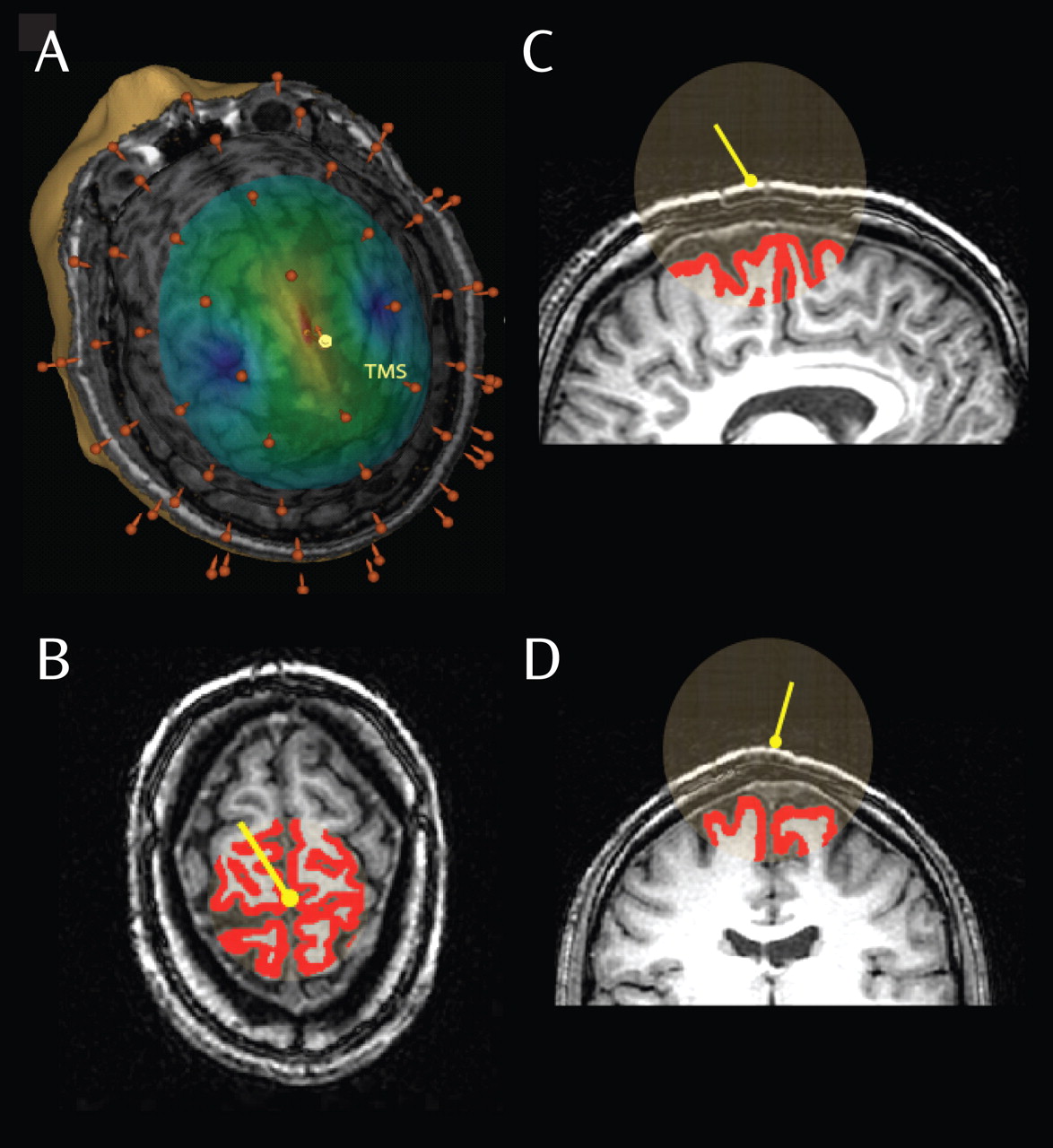
TMS can be combined with high-density EEG to directly probe thalamocortical circuits without engaging the subject in sensory, cognitive, or motor tasks. In the present study, we investigated the EEG responses in schizophrenia patients and healthy comparison subjects after TMS of the premotor cortex. We found a marked decrease in the amplitude and synchronization of gamma oscillations in schizophrenia patients, which occurred within the first 100 msec after TMS. The dampening of gamma oscillations was prominent in a fronto-central region and was associated with an impairment of effective connectivity, as revealed by source localization of TMS-evoked cortical activation. These findings suggest that frontal thalamocortical circuits in individuals with schizophrenia may be impaired in the capacity to effectively produce and synchronize gamma rhythms.
Gamma Band Abnormalities in Schizophrenia
Deficits in evoked gamma activity have recently been reported in several studies involving schizophrenia patients. In particular, three studies
(8,
19,
20) that used an oddball paradigm and one study
(4) that used a cognitive task found a decrease in evoked gamma responses in the frontal regions in schizophrenia patients relative to healthy comparison subjects. Additionally, two investigations that used steady-state auditory stimulation found deficits in gamma synchrony and power in the auditory cortex in individuals with schizophrenia
(1,
2) . Furthermore, two studies using gestalt stimuli revealed a reduction in the gamma synchronization of the visual cortex in schizophrenia patients relative to healthy comparison subjects
(7,
21) . The deficits reported in these studies were found in different schizophrenia subtypes and were not correlated with duration of illness and medication status.
While these studies provide intriguing evidence that EEG gamma-band abnormalities may represent a common feature in schizophrenia, there are several open questions that deserve investigation. For example, are gamma abnormalities confined to evoked responses, or do they reflect alterations in baseline gamma activity? Where do gamma abnormalities originate within the neuronal relays that are engaged by sensory stimulus? Additionally, do gamma abnormalities primarily originate in sensory pathways and cortices, or are they intrinsic to other brain areas? Finally, do gamma abnormalities reflect an intrinsic alteration of brain circuits, or do they represent indirect consequences of other factors, such as impaired attention to stimuli?
In the present study, we attempted to address some of these questions by taking advantage of a combined TMS/high-density EEG protocol. TMS is a powerful, noninvasive tool that can be used to directly activate the cerebral cortex
(22) . TMS has already been used in schizophrenia research to investigate changes in the excitability and inhibition of the primary motor cortex through changes in evoked muscle responses
(23) . In our study, TMS was used, with high-density EEG, to directly examine its effects on thalamocortical circuits in individuals with schizophrenia.
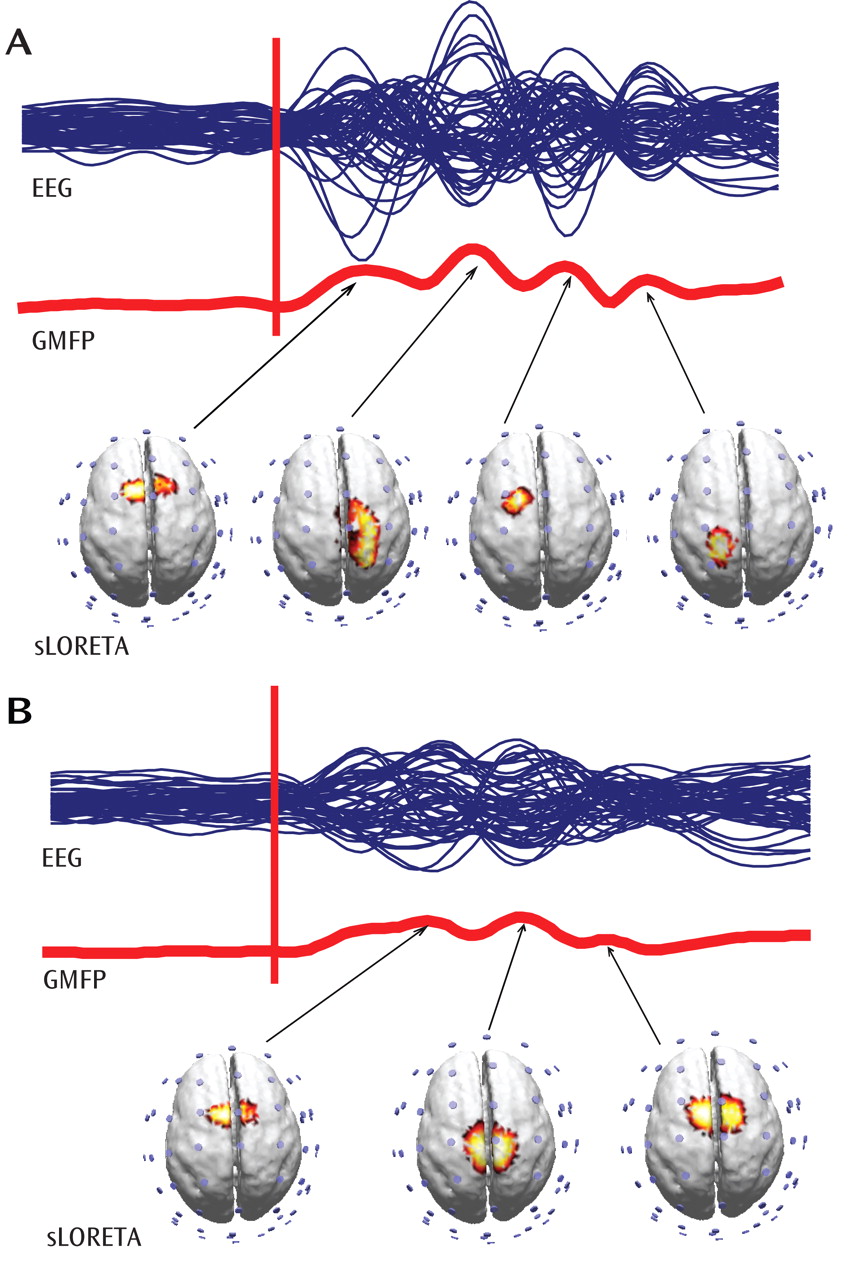
An initial finding was that TMS of the premotor cortex (measured using TMS evoked cortical potentials) showed significant differences between healthy comparison subjects and schizophrenia patients, while TMS of the motor cortex (measured using motor threshold) revealed no difference between the two groups. This result is notable, given that motor threshold and TMS evoked cortical potentials reveal different aspects of cortical excitability. Motor threshold is a measure of cortical excitability as reflected by corticospinal circuits. In contrast, TMS-evoked EEG responses reflect the excitability of stimulated cortical neurons as well as the excitability of connected cortico-cortical and cortico-thalamocortical circuits. The activation of these circuits results in the generation of EEG-recorded oscillatory activity, especially in the fast (gamma) frequency ranges.
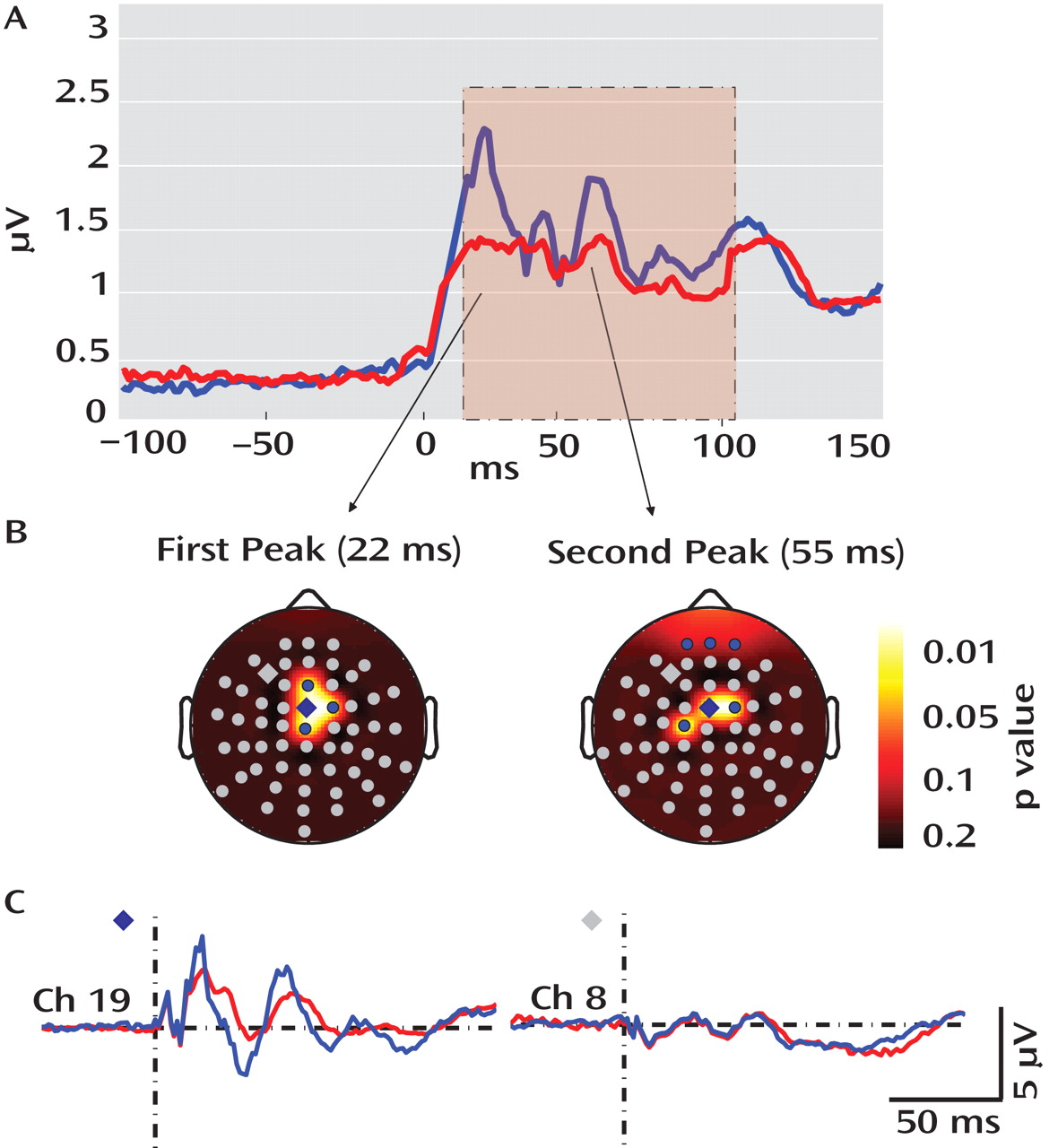
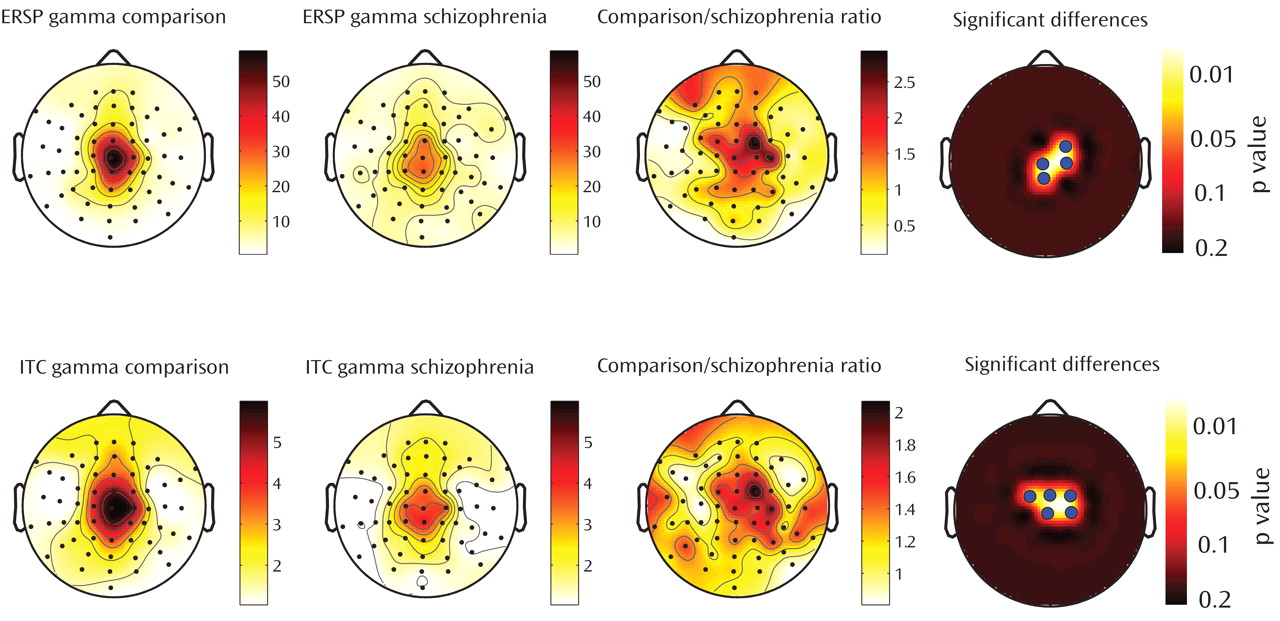
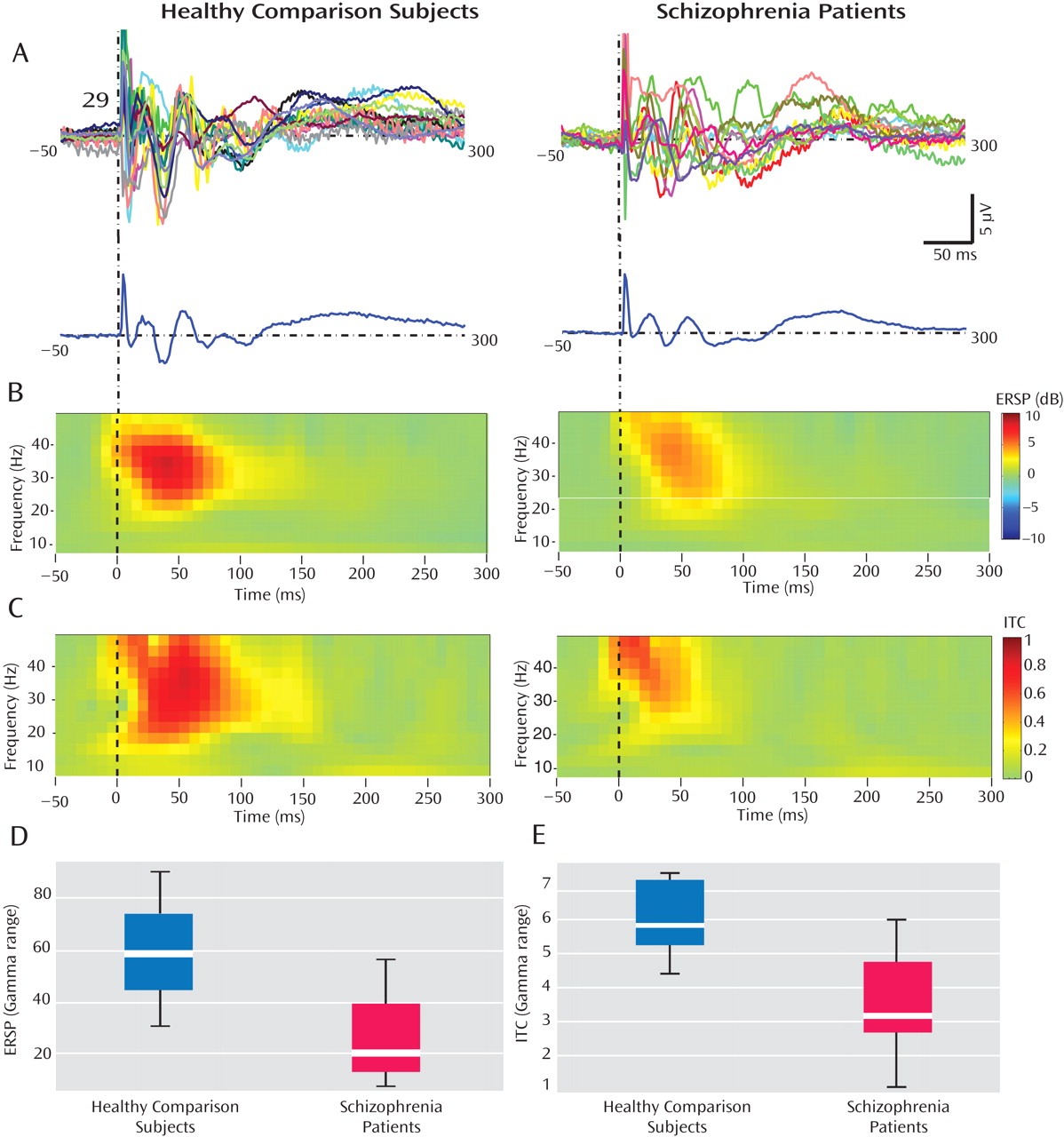
These findings, establish that gamma-band abnormalities in schizophrenia, first reported in sensory-evoked response studies, can also be demonstrated when directly stimulating the cerebral cortex. Relative to healthy comparison subjects, gamma responses in schizophrenia patients were markedly attenuated within the first 100 msec after TMS. Possible explanations for these gamma deficits are 1) a reduction in the amplitude of the TMS-evoked responses across trials or 2) a reduction in the synchronization of the TMS-evoked responses across trials. We therefore examined these two aspects of the responses independently, using event-related spectral perturbation and intertrial coherence analyses. We found that event-related spectral perturbation gamma values, which measure gamma amplitude following TMS (regardless of the phase), were significantly decreased in schizophrenia patients. In addition, the intertrial coherence gamma values, which measure gamma synchronization (regardless of signal amplitude), were also reduced in the schizophrenia group. In particular, intertrial coherence values in a fronto-central area close to the TMS coil yielded a ≥80% nonoverlap between schizophrenia patients and healthy comparison subjects. This finding is especially notable, since it has been suggested that gamma synchrony underlies core deficits in schizophrenia
(2,
4,
6,
24) . Several investigations have also suggested that gamma synchrony may correlate with clinical parameters, including negative symptoms or duration of illness. In our study, we did not find any significant correlation with age, severity of symptoms, and medication dose, perhaps because of the limited schizophrenia sample size (N=16), which was determined by the complexity of the TMS/high-density EEG approach.
The present results not only add to the evidence suggesting that gamma abnormality might be a robust feature of schizophrenia, but they also address some of the issues unanswered in previous studies. First, our study design allowed us to compare, within the same subjects, spontaneous EEG activity (specifically in the gamma range) with the gamma responses evoked by TMS. Consistent with other recent investigations
(25,
26), we found no differences in the spontaneous gamma activity in schizophrenia patients and healthy comparison subjects. However, in these same subjects, we found a prominent decrease in TMS-evoked gamma activity. Therefore, it appears that the underlying deficits, whatever their nature, become evident when the relevant brain circuits are challenged by phasic stimuli that engage high-amplitude gamma oscillations, but not necessarily under tonic conditions.
Second, whereas changes in cortical gamma response that are triggered by peripheral stimuli could result from a weakened input because of alterations in a number of subcortical neuronal relays, our measurements clearly point to a primary involvement of cortical circuits. As measured by the navigated brain stimulation system and the volumetric analysis, the cerebral cortex was perturbed with the same amount of energy in healthy comparison subjects and schizophrenia patients. Thus, the altered response observed in individuals with schizophrenia cannot be explained by a weakened volley to cortical circuits. This notion is strengthened by the observation that even higher doses of TMS in schizophrenia patients did not result in a recovery of evoked fast oscillations.
Third, while sensory stimuli do not activate the frontal cortex directly, by using TMS we directly stimulated a frontal area: the right premotor cortex. This central midline area was selected primarily because it can be stimulated without eliciting scalp muscle activations, a source of EEG artifact and subject discomfort. The choice of this area was also based on evidence of premotor cortex deficits in schizophrenia. In particular, three recent imaging studies have shown a reduction in the volume
(27) and functional connectivity
(28,
29) of the premotor area in schizophrenia patients relative to healthy comparison subjects. Together, the role of frontal areas is particularly relevant in schizophrenia research. Several imaging studies have reported reduced metabolism in the frontal areas in both chronic and first-episode schizophrenia patients, suggesting that this deficit is a core feature of schizophrenia
(30) . The finding of frontal gamma response deficits reported in the present study is consistent with the notion of reduced frontal metabolism in individuals with schizophrenia
(30) . Specifically, to our knowledge, the TMS/high-density EEG measurements in this study provide the first direct electrophysiological evidence of a primary deficit in frontal areas in generating and synchronizing gamma oscillations.
Finally, the TMS-induced effects on brain activity are certainly not independent of the brain state at the time of stimulation. However, TMS can be performed without requiring active participation of the subject. Thus, it is reasonable to assume that the TMS-evoked gamma response deficits found in individuals with schizophrenia are not dependent on the ability or motivation to perform cognitive tasks but rather reflect intrinsic alterations of specific brain circuits. In contrast, studies using oddball or gestalt stimuli necessarily require some degree of cognitive involvement of the subject. This is particularly relevant because cognitive aspects, such as attention and motivation, are difficult to control in experimental conditions and are known to be confounding factors in the interpretation of response deficits in schizophrenia.
Brain Circuits Underlying Gamma Oscillation Deficits in Schizophrenia
The reduction of TMS-evoked gamma oscillations in schizophrenia raises the question of which brain circuits underlie these deficits. The three most likely candidates are 1) gamma-aminobutyric acid (GABA)-ergic cortical interneurons, 2) cortico-cortical excitatory connections, and 3) cortico-thalamocortical loops.
GABA-ergic interneurons are known to play a major role in generating gamma oscillations. For example, mutual inhibition of local GABA-ergic cortical interneurons underlies the occurrence of gamma oscillations in a cortical region activated by repetitive stimulation
(31) . Hence, the gamma response deficits observed in our schizophrenia group, which were maximal in the area closer to the application of TMS, could reflect impairments of local inhibitory interneurons.
In our analysis, source modeling of the TMS-evoked gamma oscillations revealed that in healthy subjects the activation shifted from the premotor cortex to the right sensorimotor, left anterior premotor, and left sensorimotor regions. In contrast, the cortical activation in schizophrenia patients was much more localized, slowly shifting between premotor and motor areas. This finding is consistent with evidence from experimental data suggesting that there are defective interactions among cortical areas in schizophrenia
(32,
33) . Our results are also in agreement with several cognitive models of schizophrenia, suggesting that the cognitive symptoms of schizophrenia have a functional counterpart in impaired excitatory cortico-cortical connections
(34,
35) . Furthermore, evidence from pharmacological, modeling, and postmortem studies suggests that there are deficits in cortico-cortical glutamate transmission, mediated by
N -methyl-
d -aspartic-acid receptors, in schizophrenia
(36) .
There is increased evidence suggesting that the thalamus plays a role in the generation of gamma oscillations. First, electrophysiological studies combining intracellular thalamic recordings with cortical local field potentials have shown that EEG gamma oscillations are associated with a coherent gamma oscillatory activity in thalamic and cortical neurons
(37) . Additionally, a large-scale simulation of the thalamocortical system showed that both cortical and thalamic neurons are involved in generating gamma oscillations
(38) . In particular, this model revealed that cortico-thalamocortical loops, after lesions of cortico-cortical connections, can sustain gamma oscillations over the cortical depth and the thalamus. Moreover, intracranial recordings in humans
(39) have revealed abnormalities in the firing patterns of thalamic neurons in several neurological diseases that are characterized by the disruption of gamma oscillations and by the appearance of low frequencies at the EEG/magnetoencephalogram level
(40) . Intriguingly, involvement of thalamocortical circuits in schizophrenia has also been suggested in the results of high-density EEG recordings during sleep
(10), another approach that does not require any cognitive involvement by the subject. That study found that schizophrenia patients had deficits in sleep spindle activity, an oscillatory rhythm generated by the interplay between reticular and relay thalamic nuclei and amplified by thalamo-cortico-thalamic interactions. Thus, converging lines of evidence point to a dysfunction in thalamocortical circuits as a core deficit in schizophrenia.