Despite important advances in pharmacological treatments for bipolar disorder, a high proportion of patients remain highly symptomatic between episodes, and the risk of relapse over 5 years is high
(1) . This is the case even in patients receiving treatment with both pharmacological and psychological interventions
(2) . Given these circumstances, there is a critical need to identify the mechanisms that sustain interepisode dysfunction and trigger relapse and to develop methods to target these mechanisms with specific, empirically derived treatments. Sleep disturbance is among the most prominent correlates of mood episodes and inadequate recovery, yet sleep has been minimally studied in ways that integrate mechanistic understanding and treatment.
Methodological Considerations
Several methodological issues should be clarified from the outset. First, sleep disruption and circadian rhythm disturbance have both been implicated in bipolar disorder. However, they are not identical processes. Although there is an interaction between them, the amount and patterning of sleep depends on many factors, of which the circadian rhythm is only one. Much of the literature on bipolar disorder tends to blur this distinction. The most direct measure of the circadian system is melatonin, which is released by the pineal gland. The most direct measure of the sleep system is the combination of subjective and objective estimates of sleep. Because these systems are interconnected, each is influenced, at least to some extent, by the other.
Second, a distinction can be drawn between insomnia (a subjective perception of inadequate sleep) and sleep deprivation (an objectively measured decrement in sleep). Few studies have adopted a multimethod approach to measuring sleep in bipolar disorder so as to capture both insomnia and sleep deprivation. In this review, I use the umbrella term “sleep disturbance” to encompass both of these manifestations of sleep disturbance as well as other types of sleep disturbance that are characteristic of bipolar disorder, particularly hypersomnia.
Finally, given that research on a medication-free group of bipolar patients is often unfeasible, unethical, and unrepresentative, most studies are based on participants who are taking medications prescribed for bipolar disorder. Several of these medications have sedating or alerting effects and may alter sleep architecture. Strategies for reducing the potential confounding effects of medications are briefly discussed below.
Bipolar Disorder and Sleep Disturbance Often Coexist
Sleep in Episodes
DSM-IV-TR lists three bipolar disorder-related diagnoses: bipolar I, bipolar II, and cyclothymia. Sleep disturbance is listed as a symptom of each; reduced need for sleep is a symptom of manic and hypomanic episodes; and insomnia or hypersomnia are listed as symptoms of major depressive episode.
Shortcomings of the Diagnostic Criteria
Several aspects of the sleep-related diagnostic criteria for bipolar disorder create problems for researchers. For a manic episode, “reduced need for sleep” is not operationalized. Should this be a subjective judgment made by the patient? Should quantitative criteria be adopted, such as the example given in DSM-IV-TR, “feels rested after only 3 hours of sleep”? For a major depressive episode, it is not clear how “insomnia or hypersomnia nearly every day” should be operationalized. Does the patient need to meet DSM-IV-TR criteria for insomnia or hypersomnia most nights for 2 weeks (the duration requirement for the mood symptoms)? What would “nearly every day” equate to? Or should the patient meet the full DSM-IV-TR criteria for insomnia and hypersomnia? The latter both require a duration of 1 month. The hypersomnia criteria are also vague on a critical point, namely, how much sleep would qualify as “excessive sleep.” These shortcomings likely arise from the absence of data related to sleep in bipolar disorder.
Sleep Disturbance in Bipolar Episodes
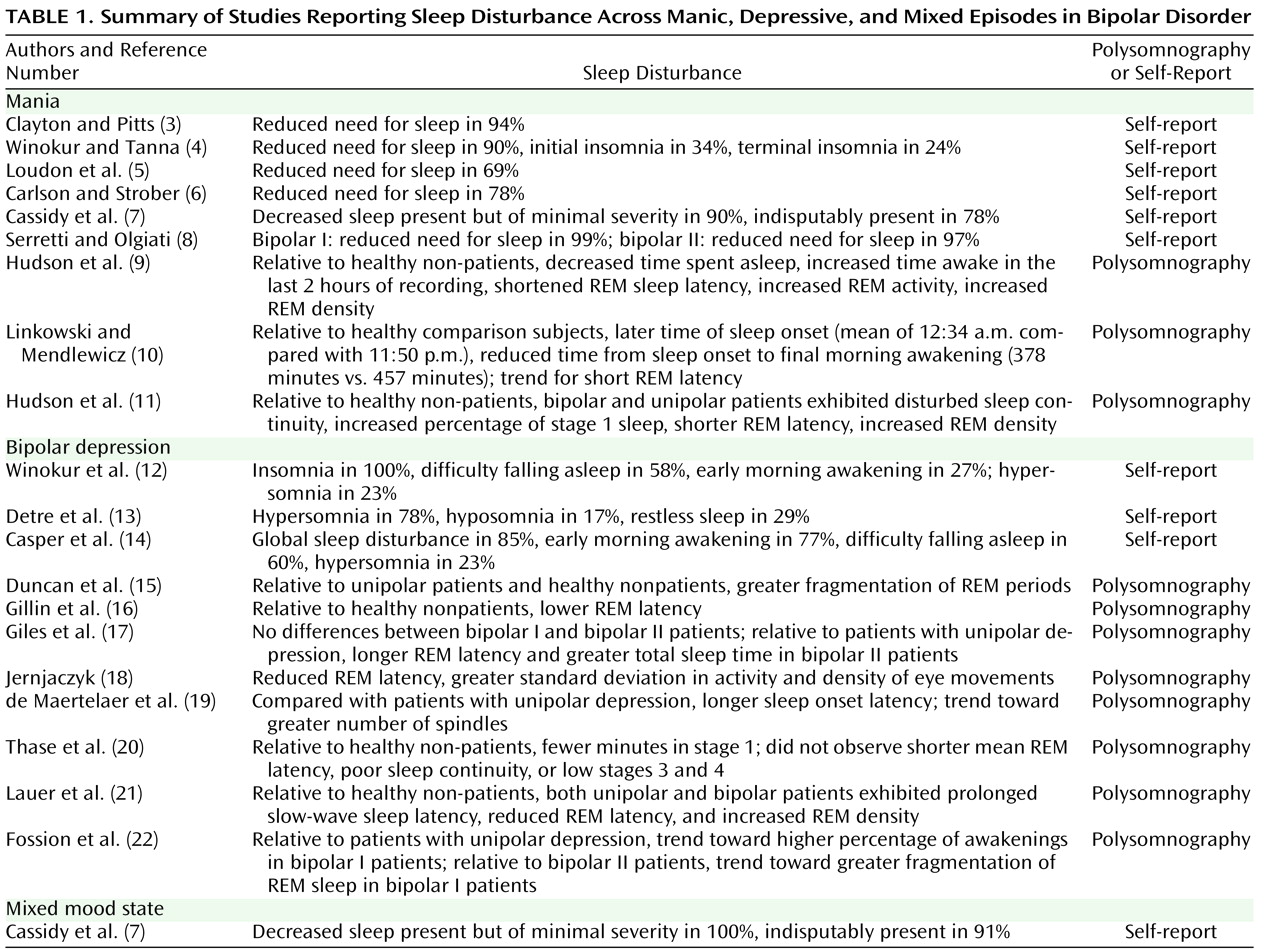
The sleep symptoms are one of a choice of symptoms that may be present for any of the bipolar diagnoses (i.e., they are not required for diagnosis). Hence, we need to establish the proportion of patients who experience sleep problems. The data available to address this question are summarized in
Table 1 . As shown in the first part of the table, during episodes of mania, the majority of patients (69%–99%) experience reduced need for sleep. Polysomnography in a sleep laboratory, the current gold-standard method for assessing sleep disturbance, was conducted in some studies. While the sample sizes for these studies were small, an association between REM sleep and mania was evident across most. This is interesting because REM sleep has been associated with affective functioning
(23) .
Studies of sleep during bipolar depression are summarized in the second part of
Table 1 . The rates of hypersomnia vary between 23% and 78%, and the rates of insomnia vary considerably, with one study reporting insomnia in 100% of depressed bipolar patients. Across the polysomnography studies, a remarkable lack of consensus is evident
(23) . Only one study was identified that monitored sleep during a mixed episode (third part of
Table 1 ); reduced need for sleep was the common sleep disturbance.
Interepisode Sleep
Significant sleep disturbance is also a feature of the period between episodes. In one study, the sleep pattern exhibited by the bipolar group more closely resembled that of an insomnia comparison group than a group of subjects with normal sleep
(24) . In another study, Jones et al.
(25) observed that the bipolar group exhibited more fragmentation of the sleep/wake rhythm and less day-to-day stability relative to comparison subjects. Millar et al.
(26) reported longer sleep onset latency and more night-to-night variability relative to comparison subjects. In the same study, a combination of actigraphy-scored variability in sleep, subjectively estimated sleep onset latency, and subjective sleep duration identified disorder status in 84% of subjects. Sitaram et al.
(27) reported higher REM density during the first REM episode and higher percentage of REM sleep in their bipolar group relative to healthy comparison subjects. Finally, Knowles et al.
(28) observed more shifts to stage I and more awake or movement time relative to comparison subjects.
Is Sleep Disturbance a State or a Trait?
Is sleep disturbance in bipolar disorder a stable feature in and out of episodes (i.e., trait-like), or does it vary across mood states (i.e., state-like)? Longitudinal data are not available to answer this question. However, taking evidence across studies, it appears that although sleep is significantly impaired during the interepisode period, sleep disturbance escalates just before an episode and worsens still further during an episode.
Focus on REM or Non-REM Sleep, or Both?
Given the findings across the studies published to date (summarized in
Table 1 ), it is not yet clear which aspects of sleep architecture will be of greatest importance in bipolar disorder. This is in contrast to the unipolar depression literature. Abnormalities in the distribution of REM and non-REM sleep, particularly a reduction of REM latency, have been consistently observed in unipolar depression
(29) . Given the intriguing association between REM sleep and mood regulation in healthy subjects
(30) and in unipolar depression
(23), as well as the existing data in bipolar disorder (
Table 1 ), it seems likely that REM sleep will be of primary interest in the context of bipolar disorder.
Does Sleep Disturbance Matter?
The data indicating pervasive sleep disturbance in bipolar disorder are of considerable concern for at least three reasons. First, sleep disturbance impairs quality of life. In the absence of bipolar disorder, poor sleep is known to have significant negative psychosocial, occupational, health, and economic effects
(31) . For example, relative to good sleepers, individuals with chronic sleep disturbances report more psychological distress and impairments in daytime functioning, take sick leave more frequently, and utilize more health care resources. Poor sleep has adverse consequences for mood, motivation, and cognitive functioning
(31) . It seem highly likely that these negative sequelae of poor sleep have significant functional consequences in individuals with bipolar disorder.
Second, sleep disturbance contributes to relapse. Multiple lines of evidence suggest that sleep disturbance contributes to relapse in bipolar disorder. While any one study alone does not provide strong evidence, the convergence of results is compelling:
Prodromes of episodes
In a systematic review of 73 reports of prodromal symptoms in bipolar disorder and unipolar depression, Jackson et al.
(32) found that the majority of patients (over 80%) were able to identify early symptoms. In patients with bipolar disorder, sleep disturbance was the most common prodrome of mania and the sixth most common prodrome of depression.
Sleep deprivation in bipolar patients
A small handful of experimental studies and case studies have reported that induced sleep deprivation is associated with the onset of hypomania or mania in a proportion of patients (e.g., reference
33 ). In a study of 206 depressed bipolar patients, Colombo et al.
(34) treated patients with one night of total sleep deprivation followed by either a recovery night or one of several medications (lithium salts, fluoxetine, amineptine, or pindolol). The results indicated that 4.85% of patients switched into mania and 5.83% switched into hypomania. It seems possible that with more than one night of full or partial sleep deprivation, the proportion of patients relapsing might be much greater.
Prospective monitoring of sleep and mood in bipolar disorder
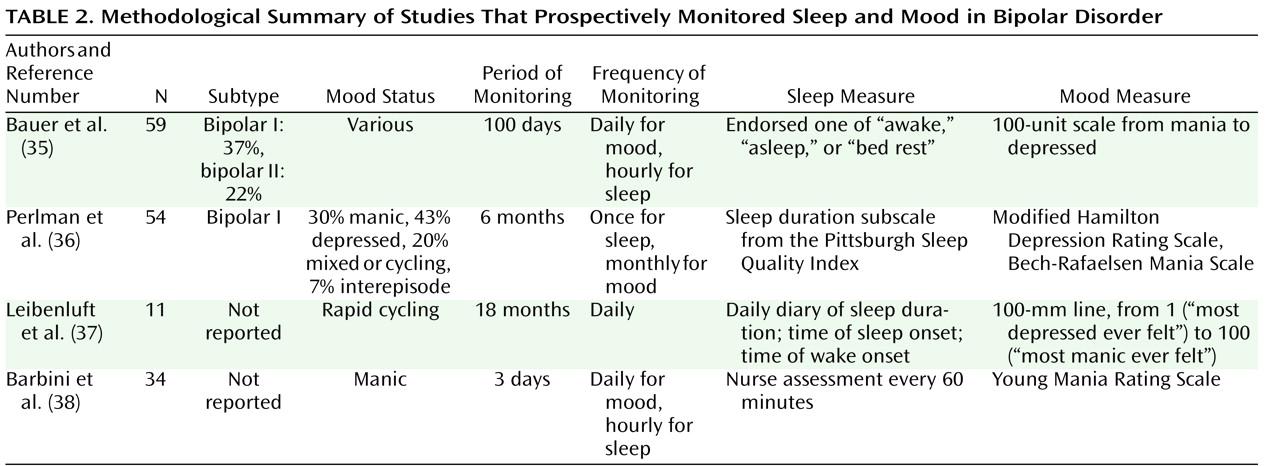
Table 2 provides a methodological summary of four studies that prospectively monitored sleep and episode status. The study by Bauer et al.
(35) found that a decrease in sleep or bed rest was followed by a shift toward hypomania or mania the next day and that an increase in sleep or bed rest was followed by a shift toward depression the next day. The latency between the shifts tended to be about 1 day. Perlman et al.
(36) found that shorter sleep duration predicted greater depressive symptoms but not manic symptoms. The Leibenluft et al.
(37) study found that shorter sleep duration predicted mania or hypomania the next day; this association was less consistent for depression. Finally, Barbini et al.
(38) observed that shorter sleep duration was associated with higher levels of manic symptoms (as reflected in scores on cooperation and irritability scales) the next day. These studies all report an association between sleep disturbance and mood, although the nature of the association—particularly whether it is stronger for manic or depressive symptoms—is less consistent. This variation may be related to differences across the studies in the measures used and the time frames studied.
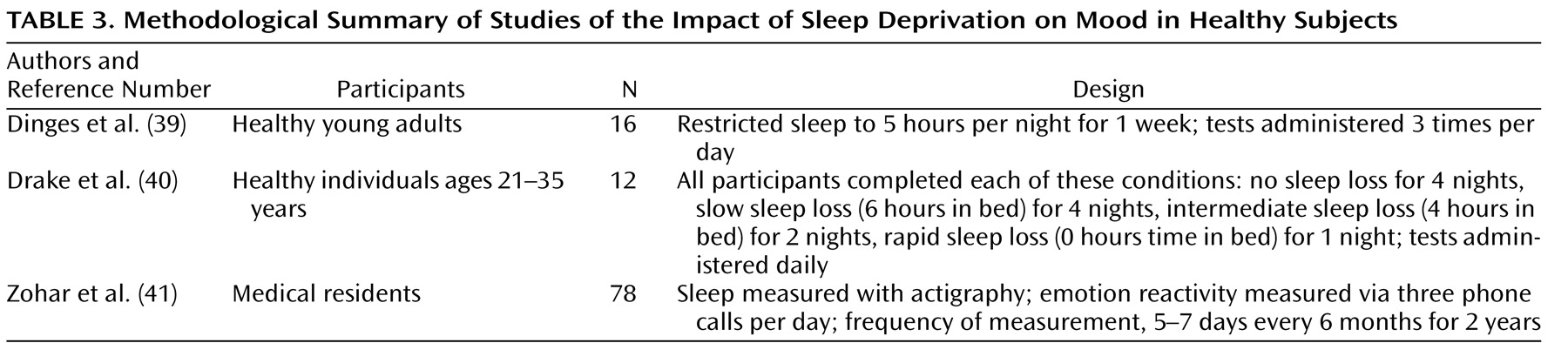
The third reason for concern about pervasive sleep disturbance in bipolar disorder is that sleep is critical for affect regulation. The three studies summarized in
Table 3 examined the hypothesis that mood is adversely affected by sleep deprivation. Dinges et al.
(39) found that mood progressively declined as sleep deprivation accumulated throughout the week. Drake et al.
(40) compared the mood responses of participants across four sleep deprivation conditions. Greater mood impairment was evident in the rapid sleep loss group (0 hours in bed) as opposed to the slow, cumulative sleep loss group (6 hours in bed for 4 nights), who experienced more impairment relative to the control group (no sleep loss for 4 nights). Zohar et al.
(41) found that context is important for determining the direction of the effect of sleep disturbance on affective functioning: sleep loss appears to intensify negative emotions following a goal-thwarting event as well as to diminish positive emotions following a goal-enhancing event. Although these studies were conducted with healthy volunteers, the observed effects may be greater in patients with bipolar disorder, who presumably have a more vulnerable affect-regulation system.
Mechanisms
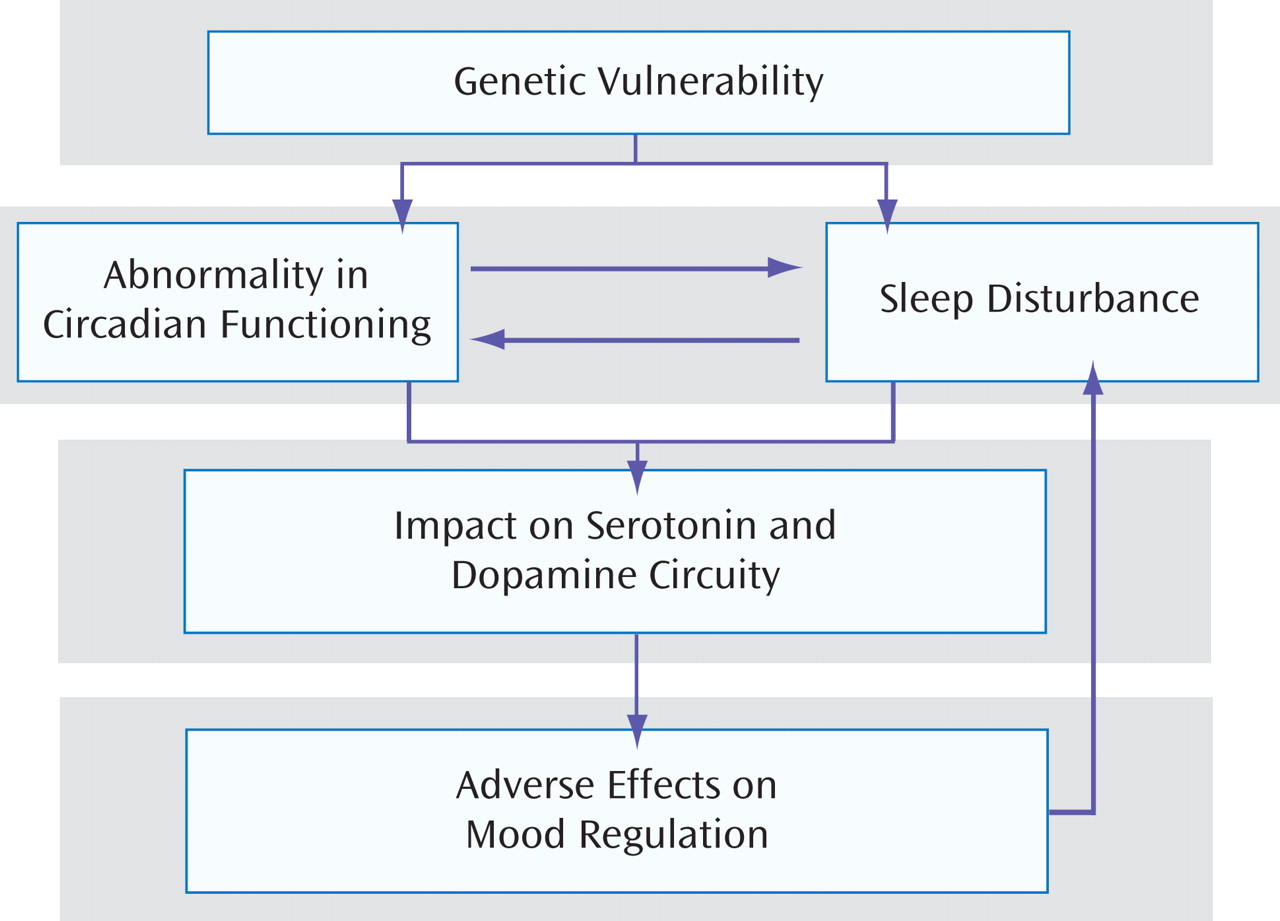
The previous section summarized evidence for the importance of sleep for quality of life and for optimal affect regulation. Moreover, the evidence suggests that sleep disturbance can trigger mood episodes in bipolar disorder. In this section, three sets of evidence are presented that address possible mechanisms by which sleep and/or circadian functioning may contribute to bipolar disorder. As
Figure 1 suggests, the mechanisms reviewed are not mutually exclusive. Indeed, they are likely to interact in complex and bidirectional ways. The groupings of evidence presented in this section on circadian versus sleep-related mechanisms could be criticized as being arbitrary given the interconnectedness between these systems. However, for clarity to emerge, it is important that we cease the current blurring of the two systems in the bipolar literature by parsing out domains of independence as well as interactions between the two systems. The criterion used to group the evidence was whether the measures or methods used were more likely to be tests of the circadian system (e.g., light, melatonin) or more likely to capture the sleep system (e.g., subjective or objective measures of sleep).
Genetic Vulnerability/Endophenotype
Bipolar disorder is known to be highly heritable. Given that specific gene loci for bipolar disorder have not yet been confirmed, it has been argued that complex psychiatric diagnoses like bipolar disorder need to be decomposed into endophenotypes for genetic analysis to progress
(42) . Several genes known to be important in the generation and regulation of circadian rhythms and the sleep system are associated with bipolar disorder, including Timeless, Clock (311 T to C), and BMal1. These associations are modest, which is consistent with the proposal that bipolar disorder is likely to be associated with multiple genes of small effect
(42) .
Circadian System
It has been proposed that patients with bipolar disorder have abnormally shifted or arrhythmic circadian systems. The term “circadian” refers to a time frame of “about 1 day” and captures an interesting feature of the circadian clock, namely, that it runs slightly longer or shorter than 24 hours. Evolution has endowed us with a biological system that is highly responsive to
zeitgebers (“timegivers”)—stimuli in the environment that cue the system so that our circadian rhythms become synchronized with the activity in the world around us. Our system is particularly sensitive to the zeitgeber light. An active process known as entrainment keeps our system aligned with external time and allows it to shift as the balance of light and dark varies across the seasons and as we travel from one time zone to another. It is an incredibly simple yet intricately orchestrated system. The “master clock” is located in the suprachiasmatic nucleus (SCN) in the anterior hypothalamus. The SCN governs all circadian rhythms and is supplemented by a large number of peripheral clocks across organs and cells
(43) . When the system is in harmony, it is fully synchronized by the SCN. But this harmony across systems can be easily lost at various junctures, including between the SCN and external time and between the SCN and different organs in the body
(43) . For the latter, each clock is differentially sensitive to zeitgebers. The SCN is very responsive to light, the clock in the liver is very sensitive to food, and clocks in muscle are sensitive to exercise. So there are many inputs that can influence and contribute to harmony and dysregulation
(43) .
In the sections below, the evidence implicating the circadian system is reviewed. Again, although any one study or line of evidence is not strong on its own, the convergent evidence is compelling.
Light
Several studies have suggested that bipolar disorder is characterized by enhanced light sensitivity. For example, in a recent study
(44) light was administered in the morning or at midday to nine depressed women with bipolar disorder. Three of the four who received light in the morning developed a mixed state, and the other responded well. Four of the five who received midday light responded well. It is possible that the greater impact in the morning reflects an increased sensitivity of the photoreceptors in the eye
(44) .
Melatonin and cortisol
Melatonin and cortisol are “hands” of the circadian clock that modulate the sleep-wake cycle. In one study, euthymic bipolar patients exhibited lower melatonin levels and a later peak time for melatonin during the night relative to a healthy comparison group
(45) . In another study, bipolar patients experiencing pure mania exhibited higher cortisol levels during the night and an earlier nadir for plasma cortisol relative to a healthy control group
(46) .
Lithium
Lithium is an effective mood-stabilizing treatment, but its mechanism of action is not certain. Given evidence across species that lithium slows down circadian periodicity and can modify circadian cycle length
(47), it may target dysregulated circadian rhythms. Indeed, in a case series of seven rapid-cycling bipolar patients, five exhibited a circadian rhythm that ran fast, and in these participants lithium slowed the rhythm
(48) .
Interpersonal and social rhythm therapy
A core concept of the social zeitgeber theory is dysfunction in the circadian system
(49) . This theory suggests that episodes of depression and mania or hypomania arise as a consequence of life events. These begin a causal cascade of processes: a life event disturbs social zeitgebers such as mealtimes and bedtimes, and these changes then derail the circadian rhythm, triggering relapse. Evidence for the various predictions of this theory is accruing, and the treatment derived from the theory—interpersonal and social rhythm therapy—has been shown to be effective in reducing relapse in bipolar disorder
(49) . One goal of this treatment is to stabilize social zeitgebers.
Total and partial sleep deprivation
A startling improvement in mood is observed in 40%–60% of depressed bipolar patients after total or partial sleep deprivation
(50) . However, symptoms of depression quickly return after the patient has slept. Two of the leading explanations implicate a circadian mechanism.
The internal coincidence model proposes that depressed patients are sleeping at the wrong biological clock time because the phase angle between the biological clock and the sleep-wake cycle is out of alignment
(51) . According to this theory, sleep deprivation is therapeutic because it prevents sleep at the critical phase. But with recovery sleep, the misalignment is reinstated.
The therapeutic effect of sleep deprivation has been proposed to increase homeostatic pressure and thereby counteract the hyperaroused state in depression
(43) . The two-process model proposes that sleep is modulated by the interaction of a homeostatic process (known as process S) and a circadian process (known as process C). It has been proposed that depression is characterized by a deficiency in the building up of process S. Sleep deprivation may cause a short-term increase in process S to normal levels
(43) . Relapse then occurs because process S returns to low levels.
Sleep
At the neural systems level, circuits involved in affect regulation and circuits involved in sleep regulation are known to interact in bidirectional ways
(52) . For example, in one study
(53), healthy participants who were sleep-deprived for 35 hours and those who had slept normally completed an emotional stimulus viewing task (100 images varying in emotional intensity) in an event-related functional MRI design. As expected, both groups exhibited amygdala activation in response to negative stimuli. However, relative to those who slept normally, those who were sleep-deprived exhibited more than 60% greater amygdala activity. This large increase was associated with a loss of activity in the medial-prefrontal cortex, which exerts top-down control on the limbic area (including the amygdala) and functions to modulate emotional responses so they are appropriate for the context. This finding suggests that sleep contributes to maintaining the connectivity between the medial-prefrontal cortex and the amygdala, which is critical for responding appropriately to emotional challenges the next day. Although the study used healthy participants, it is certainly tempting to speculate that this finding is particularly relevant to patients with bipolar disorder, whose emotion regulation system is likely to be even more vulnerable to the adverse consequences of sleep deprivation.
Studies of therapeutic sleep extension also suggest the potential importance of sleep. A promising case study showed that increasing and stabilizing sleep reduced rapid cycling
(54) . In a preliminary trial of “dark therapy” by Barbini et al.
(55), 16 bipolar patients in a manic episode were treated with 14 hours of enforced darkness for 3 consecutive days and 16 others received treatment as usual. Those who received dark therapy exhibited a decrease in manic symptoms compared with the those in the treatment-as-usual group.
Critical Link: How Do Circadian Dysregulation and Sleep Disturbance Trigger Affective Disturbance?
As already noted, although the circadian system and the sleep system are potentially separable, they are interconnected. Several researchers have implicated the serotonin and dopamine systems as the critical links between abnormalities in the sleep and circadian systems and affective functioning in bipolar disorder. Since it would be premature to cite this evidence as more pertinent to circadian versus sleep-related mechanisms, the evidence will be discussed here as likely to be relevant to both.
Evidence for the involvement of the serotonin system includes the finding that serotonin turnover increases immediately after light exposure, and the highest concentrations of CNS serotonin are in the suprachiasmatic nucleus and the raphe nucleus, part of the serotonergic pathway. In addition, there is increased sensitivity of 5-hydroxytryptamine (5-HT) neuronal firing under conditions of sleep deprivation
(43) . Finally, in patients with bipolar disorder, those who were homozygotic for the long variant of the 5-HT transporter gene were shown to have better mood amelioration after sleep deprivation than those who were heterozygotic and homozygotic for the short variant
(56) .
Evidence implicating limbic dopaminergic pathways includes the finding that administration of amineptine (a dopamine agonist) before therapeutic sleep deprivation prevented the improvement in depressive symptoms that would typically have been observed. Also, sleep deprivation seems to activate limbic dopamine pathways (indexed by increased limbic blood flow, increased dopamine D
2 receptor occupancy, and increased eye blink rates after total sleep deprivation)
(57) . Finally, a dopamine reuptake inhibitor has been shown to prevent the antidepressant effect of sleep deprivation
(58) .
Unresolved Issues
Given the data reviewed, advancement of knowledge about the role of sleep and circadian influences on sleep holds promise for providing a scientific basis for new theories of bipolar disorder and developing novel adjunctive interventions that have the potential to improve quality of life, improve affective functioning, and reduce the risk of relapse in bipolar patients. Studies in the small pool of research published to date have tended to be based on small samples and have often used suboptimal indices of sleep. More informative methods would include systematic longitudinal research of daily subjective and objective estimates of sleep following recently published guidelines
(65), as well as analyses of the macroarchitecture (e.g., sleep onset latency, REM variables, stages 1, 2, 3, and 4, and wakenings) and microarchitecture (quantitative EEG) of sleep. Data for the latter are particularly scarce in bipolar patients.
As noted earlier, a key challenge in conducting research in this area is the potential confound of mood medications that affect sleep. Strategies to manage this confound include keeping medication use constant, as long as clinically indicated, for the duration of the study; adopting within-subject or prospective designs to minimize intersubject variability in medication use; and excluding patients who are taking highly sedating medications.
Natural history data on sleep in bipolar disorder are needed to improve our understanding of the similarities and differences in sleep across mood states (euthymia, mania, depression, and mixed) and across the diagnostic subtypes (bipolar I, bipolar II, and cyclothymia). The variable presentations of “sleep disturbance” include sleeping too much (hypersomnia), difficulty getting to sleep (sleep onset latency insomnia), difficulty staying asleep (wake after sleep onset insomnia), waking up too early (early morning waking insomnia), delayed circadian phase, and difficulty regularizing the circadian cycle. Each of these may be driven by different mechanisms, and interventions for each are also likely to be different.
The mechanisms underlying the association between sleep and affective functioning in bipolar disorder are not yet understood. Some of the evidence may be accounted for in a simple framework of an escalating, bidirectional vicious circle of disturbance in mood regulation during the day interfering with nighttime sleep and the effects of sleep deprivation contributing to difficulty in affect regulation the following day (
Figure 1 ). However, the role of other systems, such as the hypothalamic-pituitary-adrenal axis and the distribution of REM and non-REM sleep, remain to be understood.
Progress is also needed to parse out the relative contributions of sleep disturbance and circadian dysregulation to bipolar symptoms. Progress on understanding the role of the circadian system in bipolar disorder is hampered by several challenges
(43) . Cortisol is influenced by stress. Melatonin is suppressed by light (even indoor room light). State-of-the-art methods for delineating the differential contributions, such as constant routine and forced desynchrony, have been developed, but these are practically challenging to carry out with patient groups.
Finally, it will be critical to consider a developmental approach. The emergence of bipolar disorder during childhood and adolescence is of particular concern because early-onset illness appears to have a more severe presentation and course. Minimal data are available on the prevalence and course of sleep disturbance in youths with bipolar disorder. Moreover, research and clinical questions related to the possible link between sleep disturbance and mood have yet to be addressed (see reference
66 for a review). Given the compelling evidence for sleep in learning and motivation, research on and development of interventions targeting sleep in these younger age groups are a high priority.