Each day in the United States, approximately 4,000 adolescents initiate cigarette smoking
(1), and data from annual surveys suggest that the decade-long decline in teenage smoking has recently slowed
(2) . Researchers consistently report that lifetime exposure to parental smoking predicts offspring tobacco use
(3 –
5) . Two issues arise in the evaluation of this literature. The first involves the manner in which smoking behavior is transmitted from parent to offspring. In nonadoptive families, genetic and environmental effects are confounded
(6) . Thus, the observation that the birth children of smoking parents have elevated rates of tobacco use does not tell us whether the basis for this association is environmental (e.g., the smoking parents model or otherwise encourage tobacco use) or genetic (e.g., the smoking parents transmit genes that increase their offspring’s liability for tobacco use). In their review of the genetic epidemiology of smoking, Sullivan and Kendler
(7) concluded that both genetic and shared familial environmental factors contribute to smoking behavior, suggesting that these factors could be acting jointly to increase the risk of tobacco use in the offspring of smokers.
A second issue concerns the specificity of the risk represented by smoking parents. Previous research suggests that smoking is, in part, an expression of a broader vulnerability to engaging in disinhibited behavior, which is distinguished more generally by undersocialized conduct and low levels of dispositional constraint
(8,
9) . Evidence from twin studies suggests that this broad vulnerability may be genetically transmitted from parent to child
(10) . From this perspective, familial resemblance for disinhibition is general rather than disorder specific. Thus, nonadoptive parents who engage in behavior like smoking may also transmit a nonspecific genetic risk that increases the likelihood that their offspring will engage in tobacco use, as well as other forms of disinhibited behavior
(11) . Of course, this risk would not be present in adoptive families.
To address these issues we used an adoption design first elucidated by Scarr and Weinberg
(12) in 1983, which offers an especially sensitive and precise test for the presence of family-level environmental influences. That is, the correlation between two individuals who are not biologically related but who have been reared together provides a direct estimate of the shared environmental effect. Genetic influences are inferred only to the extent that larger effects are demonstrated in biologically related families than in adoptive families.
In this study, we compared the effect of exposure to a nonadoptive parent who smokes, reflecting both genetic and environmental risk, with the effect of exposure to an adoptive parent who smokes, reflecting environmental risk only. To examine the specificity of the risk, we included a diverse array of adolescent outcomes, both behavioral and dispositional, that tap vulnerability to disinhibition.
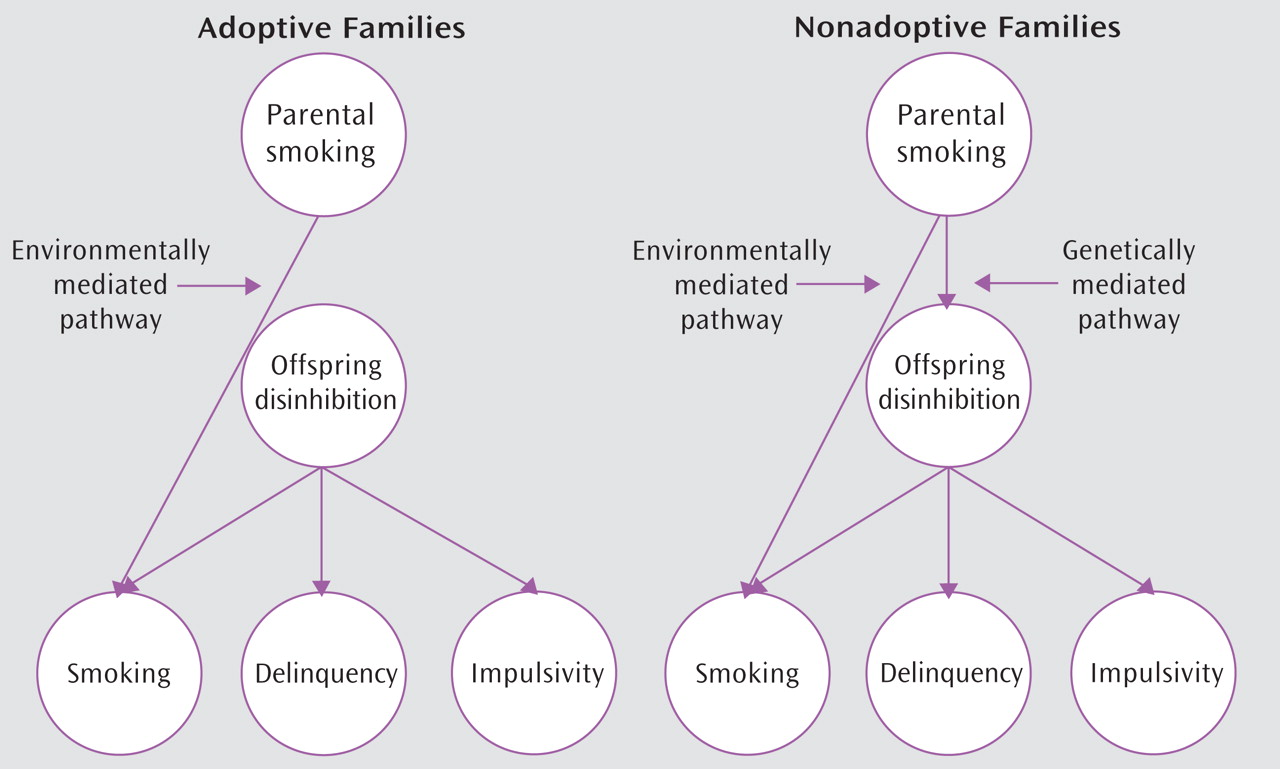
Figure 1 provides a schematic overview of our adoption design and hypotheses. We hypothesized that exposure to parental smoking represents a specific environmental risk for adolescent tobacco use. Additionally, we hypothesized that nonadoptive parents who smoke may also transmit a nonspecific genetic risk for disinhibited behavior not limited to tobacco use. Thus, we expected to observe increased tobacco use among both the nonadoptive and adoptive offspring of smoking parents and increased rates of other disinhibited behavior in the nonadoptive offspring only.
Method
Participants
The Sibling Interaction and Behavior Study is a population-based study of 617 families (409 adoptive, 208 nonadoptive), each with an adolescent sibling pair and at least one but typically two rearing parents. Adoptive families were systematically ascertained from infant placements made by three private Minnesota adoption agencies. These agencies minimally screen prospective parents, which is reflective of changes in adoption practices over the past 20 years. Prospective parents must demonstrate a commitment to raising a child, meet a modest minimum income level, and undergo a background criminal check, although a criminal record does not preclude placement. Nonadoptive families were ascertained through Minnesota birth records and selected to have a pair of siblings of comparable age and gender to the adoptive sibling pairs. All families were required to live within driving distance of the University of Minnesota.
In the adoptive families, sibling pairs were biologically unrelated to each other, although one sibling might be the biological offspring of at least one of the adoptive parents. All adoptees were placed permanently in their adoptive home before they were 2 years old (average placement age=4.7 months, SD=3.4). Information was not available on the birth parents of the adoptees. Adoptive parents provided information on the country of birth and ethnicity of participating adolescents. In the nonadoptive families, siblings were full biological siblings. Subsequent to participation, two adolescents were judged ineligible, resulting in a total sample of 1,232 adolescents, 613 mothers, and 551 fathers. A complete description of the recruitment process can be found in McGue et al.
(13) .
The analyses reported here used a subset of the full sample. Adolescents were only included if at least one parent was ever diagnosed with nicotine dependence and used tobacco within their child’s lifetime (i.e., ever exposed) or if both parents were never diagnosed with nicotine dependence nd never used tobacco within their child’s lifetime (i.e., never exposed). A total of 335 adolescents with data available on both parents were excluded based on these criteria. For an additional 112 adolescents, data for one parent were missing and the other parent did not meet the inclusion criteria. Adolescents excluded because of missing data did not significantly differ from those included on number of disruptive disorder symptoms or measures of lifetime substance use. The final sample included 785 adolescents from 402 families, whose mean age was 14.9 years (SD=1.9). Of these, 414 (53%) had at least one parent who was nicotine dependent and used tobacco during the adolescent’s lifetime. Ninety-three adolescents (23%) were exposed by their mother only, 229 (55%) were exposed by their father only, and 92 (22%) were exposed by both parents. Fifty-nine percent of the sample was adopted, with 66% of these adoptions originating from South Korea; 94% of the nonadopted adolescents were Caucasian.
Procedure
Families were assessed in our laboratory using a research protocol approved by the University of Minnesota Institutional Review Board. Upon arrival, a complete description of the study was provided, followed by written informed consent from parents and assent from minor offspring. All family members were interviewed simultaneously, each in a separate room by a different interviewer. Interviewers had a master’s or bachelor’s degree in psychology, participated in intensive training, passed written examinations, and satisfied proficiency criteria. Adolescent participants also completed self-report questionnaires.
Measures
The adolescent outcome battery incorporated diverse indicators of vulnerability to disinhibition, including measures of delinquent behavior, antisocial attitudes, aggressive orientation, harm avoidance, substance use, and disruptive behavior disorders. We also assessed nicotine dependence in the parents.
Self-reports of behavioral deviance and substance use
Adolescent participants completed the Delinquent Behavior Inventory
(14), a 36-item checklist of minor (e.g., skipping school) and more serious (e.g., using a weapon in a fight) delinquent behaviors (α=0.89). Adolescent participants also completed two 8-item scales assessing the cognitive factors that underlie delinquent behavior: aggressive orientation (e.g., “If I did not like someone, I might try to hurt him or her just for the heck of it”; α=0.87) and antisocial attitudes (e.g., “I see nothing wrong in trying a little beer with my friends”; α=0.87). Finally, adolescent lifetime use (at least one time without parents’ permission) of tobacco, alcohol, and marijuana was assessed via a private, computer-administered questionnaire. In our relatively young sample, lifetime use was best viewed as experimental use. Among those using substances in the last year, the modal response was less than once a month for each substance. The experimental use phenotype is especially appropriate for this age group because research has consistently shown that substance use early in adolescence is a potent predictor of later substance misuse
(15 –
17) .
Personality
The personality trait of harm avoidance was assessed using the Multidimensional Personality Questionnaire
(18), a self-report instrument assessing personality characteristics in normal populations. Those scoring high on harm avoidance tend to avoid danger, prefer safer activities, and exhibit low risk-taking behavior (α=0.84).
Clinical assessments
Participants were interviewed with the revised version of the Diagnostic Interview for Children and Adolescents (DICA-R)
(19,
20), which was modified to ensure lifetime coverage of DSM-IV childhood disorders. All questions asked of the child were asked of the mother as they pertained to the child. Disruptive behavior disorders included conduct disorder, oppositional defiant disorder (assessed without regard for the presence of conduct disorder), and attention deficit hyperactivity disorder (ADHD).
Nicotine dependence in parents was assessed with the Expanded Substance Abuse Module developed by Robins et al.
(21) as a supplement to the World Health Organization’s Composite International Diagnostic Interview (CIDI-SAM)
(22), modified for DSM-IV. Nicotine dependence was assessed over the lifetime of the parent. Parents also provided information on the tobacco products they used most heavily, the largest amount consumed, and the age at which they last used tobacco.
Adolescent and parent clinical interviews were reviewed by at least two individuals with advanced clinical training who were blind to the diagnoses of other family members. This information was used to code, by consensus, every relevant DSM-IV symptom and diagnostic criterion. For the behavior disorders assessed in adolescents, the mother and child reports were combined using the best-estimate method, in which a symptom was considered present if endorsed by either reporter
(23) . Diagnoses were considered positive if all (definite) or all but one (probable) of the DSM-IV criteria were met. Disorder symptoms were also summed to provide a quantitative index of clinically significant disruptive behavior. In the parents, nicotine dependence diagnoses were considered positive if present at the definite or probable level. Because DSM was developed to assess acute rather than lifetime psychopathology, probable diagnoses were included to minimize the likelihood of false negatives due to imperfect recall. Kappa coefficients for our assessments of childhood disruptive disorders were 0.73 (oppositional defiant disorder), 0.77 (ADHD), and 0.80 (conduct disorder). Kappa coefficients for substance use disorders, including nicotine dependence, exceeded 0.91.
Family socioeconomic status
Parents’ education level was coded on a 5-point scale (where 1=less than high school and 5=professional degree). For parents employed on a full-time basis, occupational status was coded on a 6-point reflected Hollingshead-Redlich Scale (where 1=manual laborer and 6=professional/managerial). Following procedures outlined in McGue et al.
(13), we standardized the educational and occupational status scores for each parent using the mean and standard deviation from the distribution of scores for the nonadoptive families. We then summed these standardized scores for the parents in each family to form a composite socioeconomic status indicator.
Statistical Analyses
The relationships among adoption status (adopted/nonadopted), exposure to parental smoking (ever/never), and adolescent adjustment were investigated using analysis of variance (ANOVA) for quantitative outcomes and logistic regression for categorical outcomes. The basic model included adoption status, parental smoking exposure, and their interaction as independent variables. The significance of each variable was measured against the other variables in the model using a regression approach to the analysis of genetically informative data
(24) . Adolescent age and ethnicity (Caucasian/non-Caucasian) and family socioeconomic status were included as covariates. Dependent variables included a diverse array of adolescent substance use and other disinhibitory outcomes. The correlated nature of the family data was taken into account with hierarchical linear methods as incorporated into PROC MIXED (for quantitative outcomes) and generalized estimating equations as incorporated into PROC GENMOD (for categorical outcomes) from SAS
(22) . Analyses were structured to estimate the simple main effect of exposure to parental smoking on the adopted and nonadopted adolescents separately. For quantitative outcomes, effect sizes were estimated by dividing the difference in the covariate-adjusted means by the residual standard deviation. For categorical outcomes, covariate-adjusted odds ratios and 95% confidence intervals (CIs) are reported.
Results
All (100%) of the mothers and 95% of the fathers in the exposure group reported that their most heavily used tobacco product was cigarettes. During their period of heaviest use, fathers averaged about 30 cigarettes per day and mothers almost 25 cigarettes per day. Although there was a greater proportion of nonadopted relative to adopted adolescents who were exposed to parental smoking (59% versus 48%; χ 2 =5.81, p<0.05), severity of risk indices within the exposure group were not associated with adoption status. That is, within the exposure group, nonadoptive mothers and fathers were no more likely to smoke currently than were adoptive mothers and fathers. Furthermore, adopted and nonadopted adolescents were equally likely to have been exposed to more than one smoking parent.
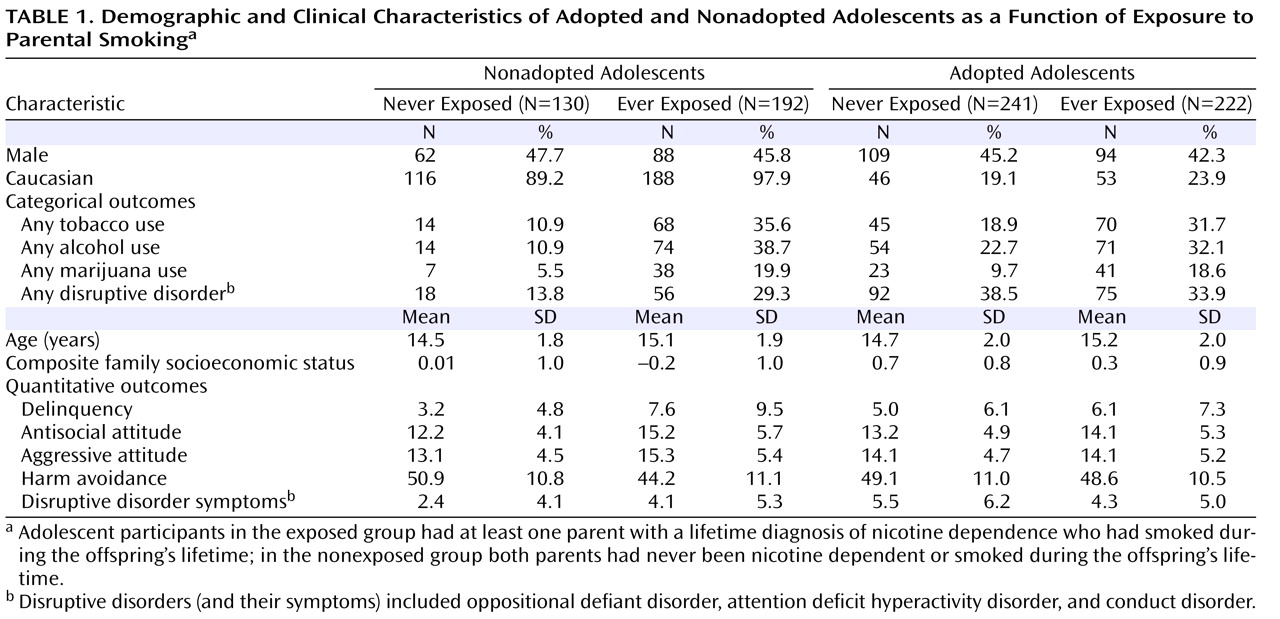
Table 1 presents demographic data on offspring ethnicity, sex, age, and family socioeconomic status.
Table 1 also includes the lifetime prevalence of tobacco, alcohol, and marijuana use and any DSM-IV disruptive behavior disorders (oppositional defiant disorder, ADHD, or conduct disorder) in exposed and nonexposed adolescents by adoption status. Finally,
Table 1 provides the means and standard deviations for quantitative variables, including self-reports of delinquency, antisocial attitudes, aggressive orientation, and harm avoidance, as well as best-estimate symptom counts for the disruptive behavior disorders.
Focusing on the demographic variables, the sex of the offspring was unrelated to exposure risk or adoption status. Ethnicity was associated with adoption status only; Caucasian participants constituted 94% of the nonadopted sample but only 21% of the adopted sample (χ 2 =134.90, p<0.001). Age at assessment was related only to exposure risk, with older adolescents more likely to be exposed to parental smoking (15.1 years old versus 14.6 years old; F=13.43, p<0.001). Family socioeconomic status was also related to exposure risk (F=28.41, p<0.001), as well as to adoption status (F=60.55, p<0.001), but not to their interaction. The mean family socioeconomic status for both exposed and nonadopted adolescents was significantly lower when compared with unexposed and adopted adolescents, respectively. Thus, all adolescent outcome variables included in our analyses were statistically adjusted for age, ethnicity, and family socioeconomic status.
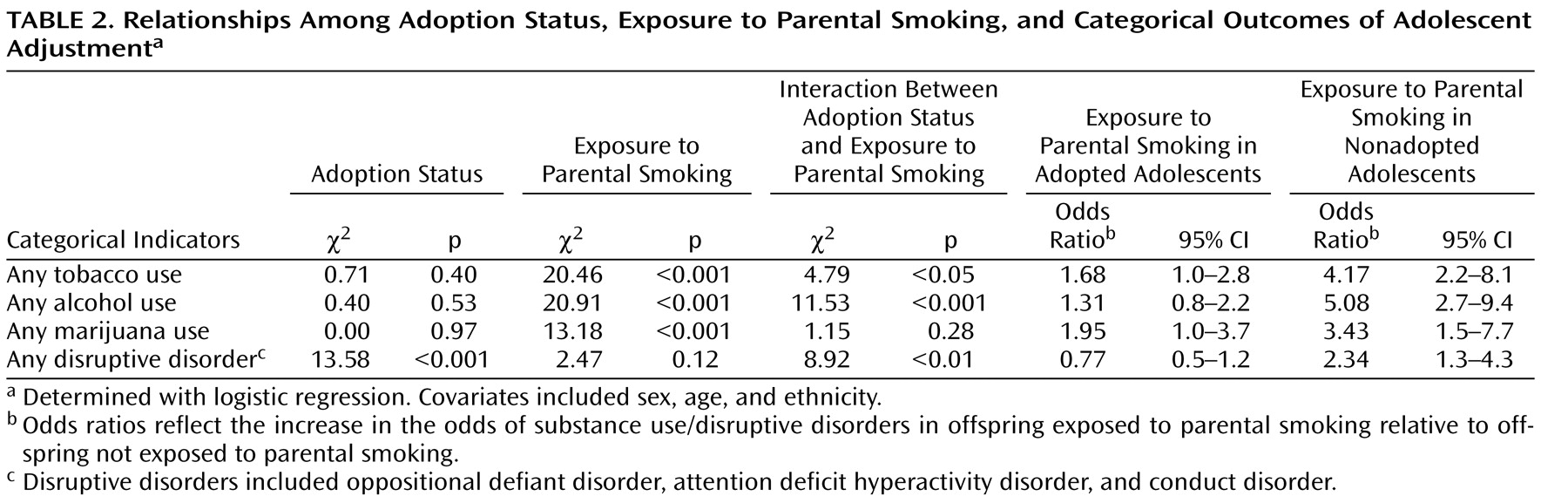
Logistic regression results, along with associated odds ratios and confidence intervals for the lifetime prevalence of tobacco, alcohol, and marijuana use, are given in
Table 2 . Across all adolescents, adoption status was unrelated to ever using any of these substances. Exposure to parental smoking was, however, associated with ever having used each of these substances. Within the adopted subsample, those adolescents exposed to parental smoking were slightly but significantly more likely to use tobacco (odds ratio=1.68) and marijuana (odds ratio=1.95). Within the nonadopted subsample, those adolescents exposed to parental smoking were significantly more likely to use not only tobacco (odds ratio=4.17) and marijuana (odds ratio=3.43) but also alcohol (odds ratio=5.08). Thus, exposure to parental smoking placed adolescents at risk for tobacco and marijuana use, whether the rearing parent was biologically related or adoptive. When the parent was biologically related, the adolescent was also at risk for alcohol use. The interaction between adoption status and exposure was significant for tobacco and alcohol use, indicating a larger exposure effect in biologically related families.
Table 2 also provides odds ratios and confidence intervals for the lifetime prevalence of any assessed disruptive behavior disorder. In contrast to the results for substance use, but consistent with other published reports
(6,
25), adoption status was associated with an increased prevalence of any disruptive behavior disorder. Importantly, this increased risk for adopted adolescents was not associated with exposure to parental smoking. However, nonadopted adolescents exposed to parental smoking were significantly more likely than those not exposed to be diagnosed with any disruptive behavior disorder (odds ratio=2.34). The interaction between adoption status and exposure was significant, indicating as before a larger exposure effect in biologically related families.
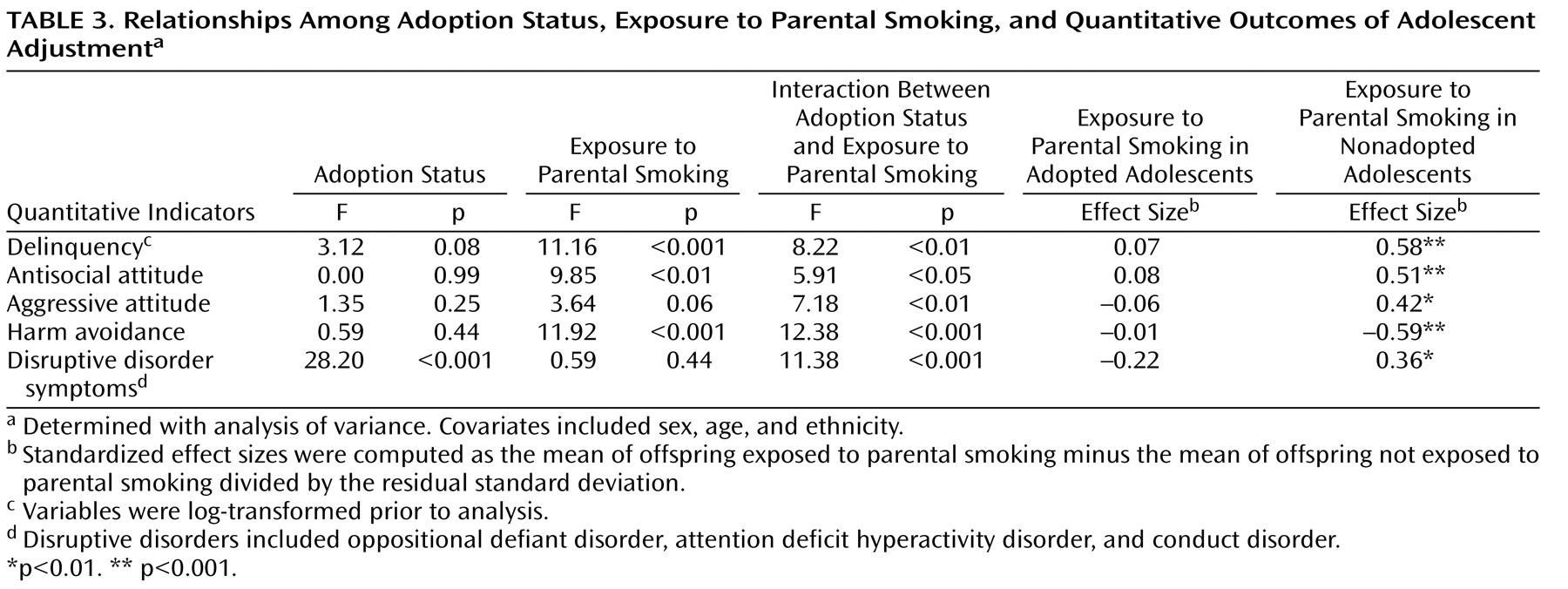
ANOVA results, along with effect size estimates for the quantitative indicators of disinhibited behavior, are given in
Table 3 . Across all adolescents, adoption status was unrelated to any quantitative indicator of disinhibited behavior save disruptive disorder symptom counts. Exposure to parental smoking was associated with three quantitative indicators of disinhibition: delinquency, antisocial attitudes, and harm avoidance. Adopted adolescents exposed to parental smoking were not at increased risk for any of the quantitative indicators of disinhibition compared with adopted adolescents who were not exposed. Conversely, nonadopted adolescents exposed to parental smoking reported significantly higher levels of delinquent behavior, more tolerance of antisocial behavior, greater acceptance of aggressive behavior, and lower levels of harm avoidance than nonadopted adolescents who were not exposed. In addition, the best-estimate symptom counts for disruptive behavior disorders were significantly higher in nonadopted adolescents exposed to parental smoking compared with those who were not exposed. Significant effect sizes were moderate, ranging from 0.36 to 0.59. Finally, the interaction between adoption status and exposure was significant for all indicators of disinhibition, indicating a larger exposure effect in biologically related families.
Discussion
Data from the adoptive families suggest that exposure to parental smoking represents an environmental risk factor for the lifetime use of tobacco and marijuana in adolescent offspring. When contrasted with data from biologically related families, however, the findings indicate that the effect of exposure to parental smoking is both larger and more diverse when adolescents and their parents share a common genetic endowment. Thus, smoking parents may provide an environment that increases their offspring’s risk of tobacco and marijuana use, and nonadoptive parents may also transmit genes that increase their offspring’s liability for disinhibited behavior, including substance use, disruptive behavior disorders, delinquency, antisocial attitudes, aggressive orientation, and preference for risk taking.
The environmental effect of exposure to parental smoking, as demonstrated in adoptive families, is consistent with studies showing a reduced risk for tobacco use in offspring of parents who have stopped smoking compared with adolescents whose parents are current smokers
(26) . Interestingly, adopted adolescents exposed to parental smoking were also at increased risk for marijuana use as well as alcohol use, although the latter did not reach conventional levels of statistical significance. This suggests that the environmental mechanism at play may not be behavioral modeling or access per se, but rather increased parental tolerance for offspring substance use or reduced authority in regulating offspring substance use when the parent is a regular smoker. Alternatively, it may reflect the role of smoking as a gateway to the use of other substances
(27) .
The larger and more diverse effects demonstrated in biologically related families are consistent with other family studies in implicating the existence of a common inherited liability across multiple indicators of disinhibition. For example, relatives of alcoholics have elevated rates of smoking and illicit drug dependence
(28), and the inherited factors that underlie conduct disorder overlap extensively with those for alcoholism
(29,
30) and drug dependence
(31,
32) . More generally, there appears to be a common familial liability to disinhibitory disorders
(10), due in large part to a general genetic vulnerability
(33) . Research in adolescent samples also confirms the existence of a common genetic liability underlying both behavioral and dispositional markers of risk
(34,
35) .
Critics of adoption studies maintain that because adoptive parents are better educated and have greater economic resources than the general population, environmental effects measured in adoptive families are greatly attenuated
(36) . Indeed, the socioeconomic status of adoptive families in our sample was significantly higher than that of the nonadoptive families. However, statistically adjusting for family socioeconomic status did not eliminate the larger and more diverse effects of parental smoking in nonadoptive families. Of course, the larger effects in nonadoptive families may be due to factors independent of socioeconomic status that are differentially present in the environments of both adoptive and nonadoptive families. In addition, the increase in disruptive behavior disorder symptoms in the adoptees could suggest that they represent a sample at high genetic risk in which the effect of exposure to parental smoking might be diminished. Although we cannot rule this out, we believe it is an unlikely explanation for our findings. In fact, we and others
(25,
37) have shown that the majority of adoptees are psychologically well adjusted with only a moderate, albeit significant, increase in risk for symptoms of disruptive disorders.
To our knowledge, only one other adoption study has examined the effect of parental smoking in adoptees
(38) . These authors reported that both adoptive and birth parent smoking were unrelated to ever smoking in adopted adults. However, this Danish registry-based sample included adoptions from 1924 to 1947, a period during which smoking was highly prevalent. Although restriction in range likely attenuated the association between parents and offspring in that study, the factors that influenced risk for smoking behavior during that period may be different from those that are operating today
(39) .
This research is not without limitations. The Sibling Interaction and Behavior Study sample is predominantly in mid-adolescence, so all participants have not yet experienced the full risk period for substance misuse. Consequently, our analysis of substance use necessarily focused on ever using rather than patterns of regular use or abuse. Further, behavioral genetic research suggests that the impact of family environmental influences on substance use and mental health outcomes diminishes with age as genetic influences increase in importance
(40 –
42) . It will be important to follow this particular sample through late adolescence and early adulthood as more of the participants initiate substance use and as others transition to regular or abusive use patterns to determine whether the effects we observed will endure.
In summary, this study presents evidence for an environmentally mediated pathway by which parental smoking increases risk specifically for substance use in adolescent offspring. The data are also consistent with a genetically mediated pathway by which nonadoptive parents who smoke may also transmit a nonspecific genetic risk to their offspring for disinhibited behavior.