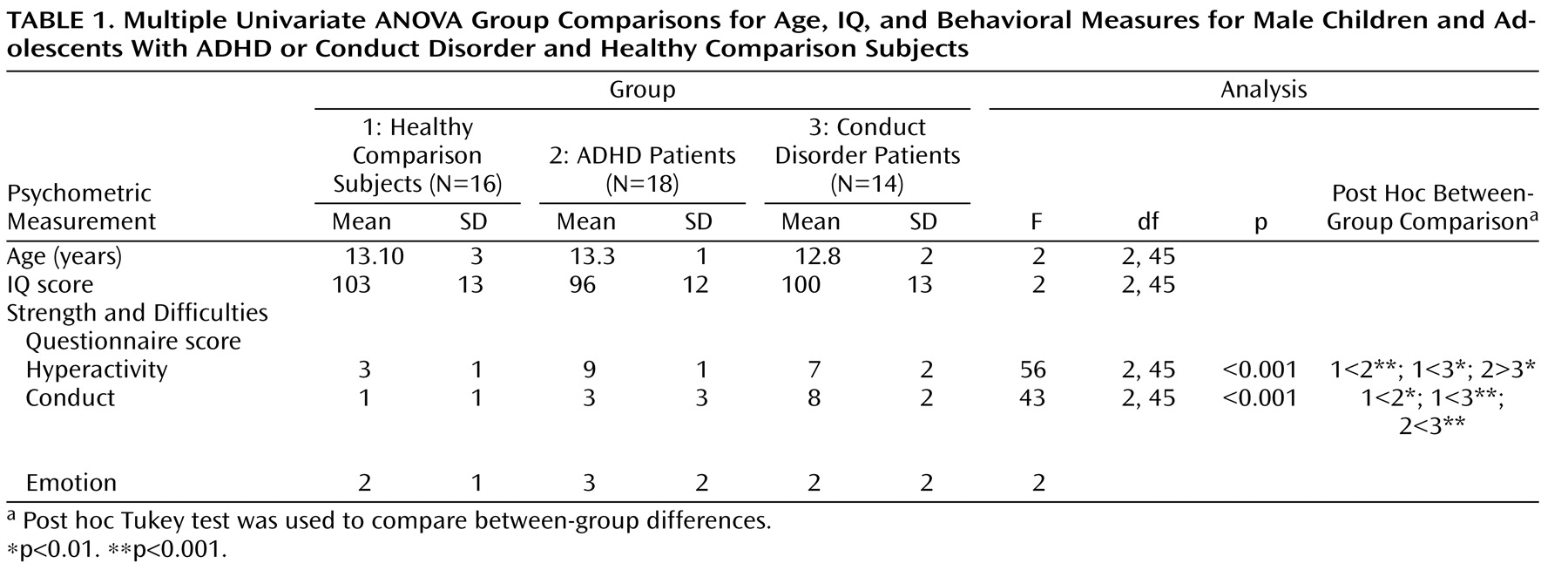
Evidence for sustained attention deficits among individuals with conduct disorder and oppositional defiant disorder is controversial and has been associated with cases of comorbidity with ADHD
(10,
11) . However, conduct disorder but not ADHD has consistently been associated with reduced sensitivity to changes in reward contingencies, specifically to punishment
(12,
13) . Consequently, attention problems among individuals with conduct disorder have been postulated as secondary to motivational deficits
(12) . Thus, neural substrates of motivation are good candidates for a distinct neurobiological basis for conduct disorder. In studies of adult antisocial behavior, neurobiological evidence has suggested that there are abnormalities in the paralimbic system, comprising orbitofrontal, superior temporal, cingulate, and limbic brain regions, which mediate the control of emotion and motivation
(14) . However, there are no fMRI studies that have examined the theory of abnormal motivation networks in children with conduct disorder. To our knowledge, only three published fMRI studies of conduct disorder in children and adolescents are present in the literature. Two of these studies examined several comorbid cases and reported 1) abnormal anterior cingulate activation during the presentation of images with negative valence
(15) and 2) reduced function of the amygdala and its interconnectivity to the orbitofrontal cortex during fearful face processing
(16) . The other study, which we conducted, reported reduced activation in the posterior cingulate and temporo-parietal regions in patients with noncomorbid conduct disorder during error processing
(17) .
Method
Subjects
Patients were 32 right-handed male children and adolescents, ages 9–16 years, who had a clinical diagnosis of either 1) conduct disorder without clinical ADHD (N=14) or 2) ADHD, combined hyperactive-inattentive subtype, without conduct disorder (N=18). Diagnoses were established using the standardized Maudsley Diagnostic Structural Interview
(23) . Recruitment was conducted via parent support groups, clinics, and advertisements. Exclusion criteria were comorbidity with other psychiatric disorders; a learning disability, reading disorder, or neurological abnormality; epilepsy; substance abuse; and previous exposure to stimulant medication. All ADHD patients achieved a score above threshold on the hyperactivity subscale of the Strength and Difficulties Questionnaire
(24) . All patients with conduct disorder also achieved a score above threshold on the conduct disorder subscale of this same questionnaire. ADHD patients were medication-naive by personal choice or were scanned prior to initial medication treatment. All patients with conduct disorder were medication naive, had early onset conduct disorder (<10 years of age), and met criteria for oppositional defiant disorder.
Healthy comparison subjects were 16 right-handed age- and IQ-matched male children and adolescents. In order to minimize differences in socioeconomic status, healthy subjects were recruited from the same local geographic area as ADHD and conduct disorder patients. Healthy subjects had no history of ADHD, conduct disorder, other mental or neurological disorder, neurotropic drug treatment, or substance abuse, and they scored below threshold on the Strength and Difficulties Questionnaire.
All participants scored above the fifth percentile (i.e., >75) on the Raven’s Standard Progressive Matrices Intelligence Questionnaire
(25) (
Table 1 ). Written informed consent/assent from parents was given, and the study was approved by the local ethics committee.
One-way analysis of variance (ANOVA) showed no group differences in age and IQ. Post hoc t tests revealed that ADHD patients scored significantly higher than conduct disorder patients and healthy comparison subjects on the hyperactivity subscale of the Strength and Difficulties Questionnaire and conduct disorder patients scored significantly higher on the conduct disorder subscale of the questionnaire relative to ADHD patients and healthy comparison subjects (
Table 1 ). The three groups did not differ significantly on the emotion symptom subscale of the questionnaire.
fMRI Paradigm: Rewarded Continuous Performance Test
A rapid mixed-trial event-related fMRI design, along with jittered and randomized presentation, was used to optimize statistical efficiency. Patients and healthy subjects practiced the task one time prior to scanning.
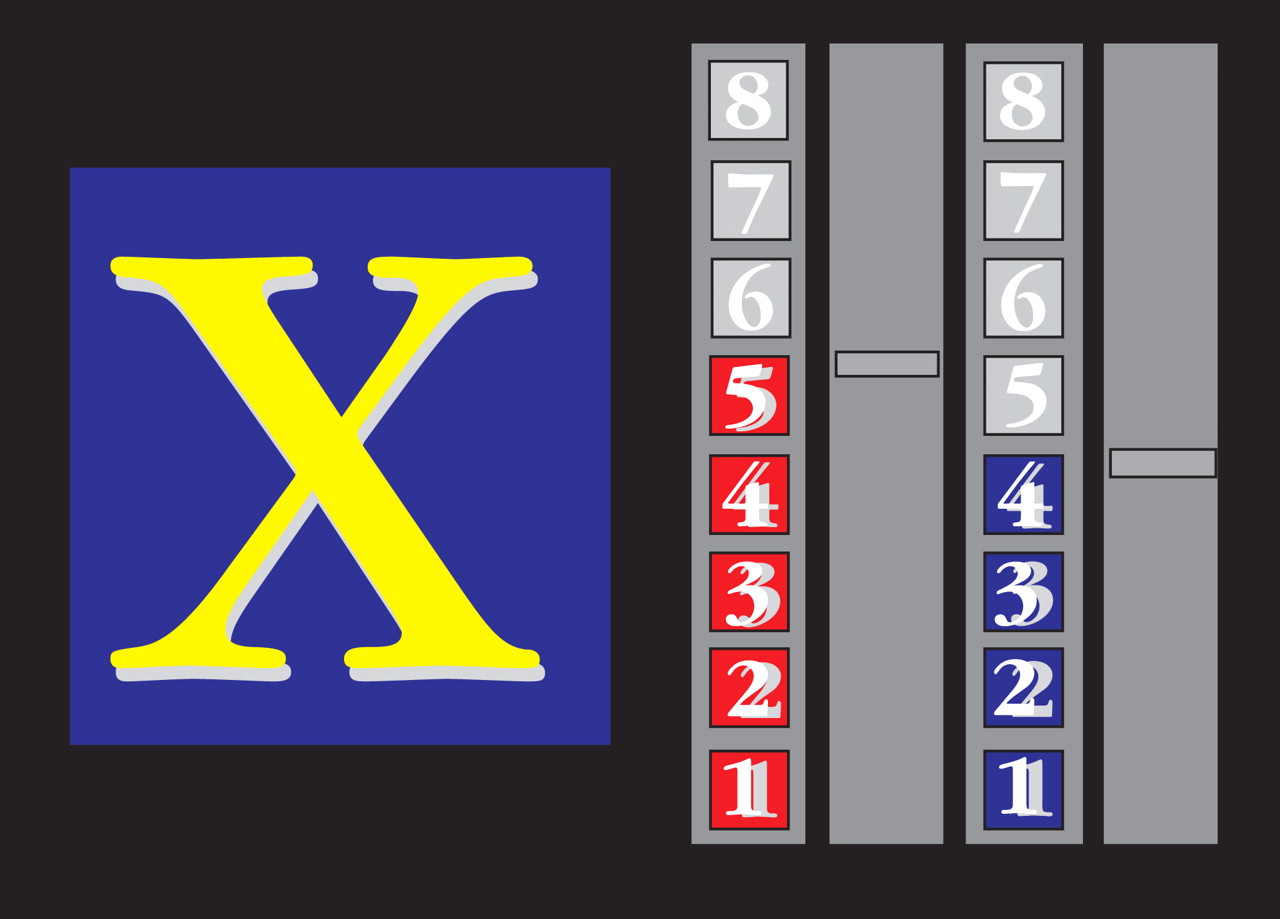
The computerized fMRI adaptation of the rewarded Continuous Performance Test consists of a stream of 416 stimuli (letters), with a presentation time of 300 msec each (mean intertrial intervals: 900 msec)
(3,
26) . Forty-eight target stimuli, letters “X” and “O,” are included. Subjects were required to respond with the right-hand button box to target letters (“X” or “O”) only. For one of the two target letters (counterbalanced across subjects), a correct response resulted in a monetary reward (£1 for every three correct responses). The amount of money earned during the task (£8 for 100% correct responses) was displayed on the right side of the screen by one of two different rising colored score bars (red, blue). For the nonrewarded target letter, the other score bar would rise by one score for every three correct responses, but no monetary reward was given (
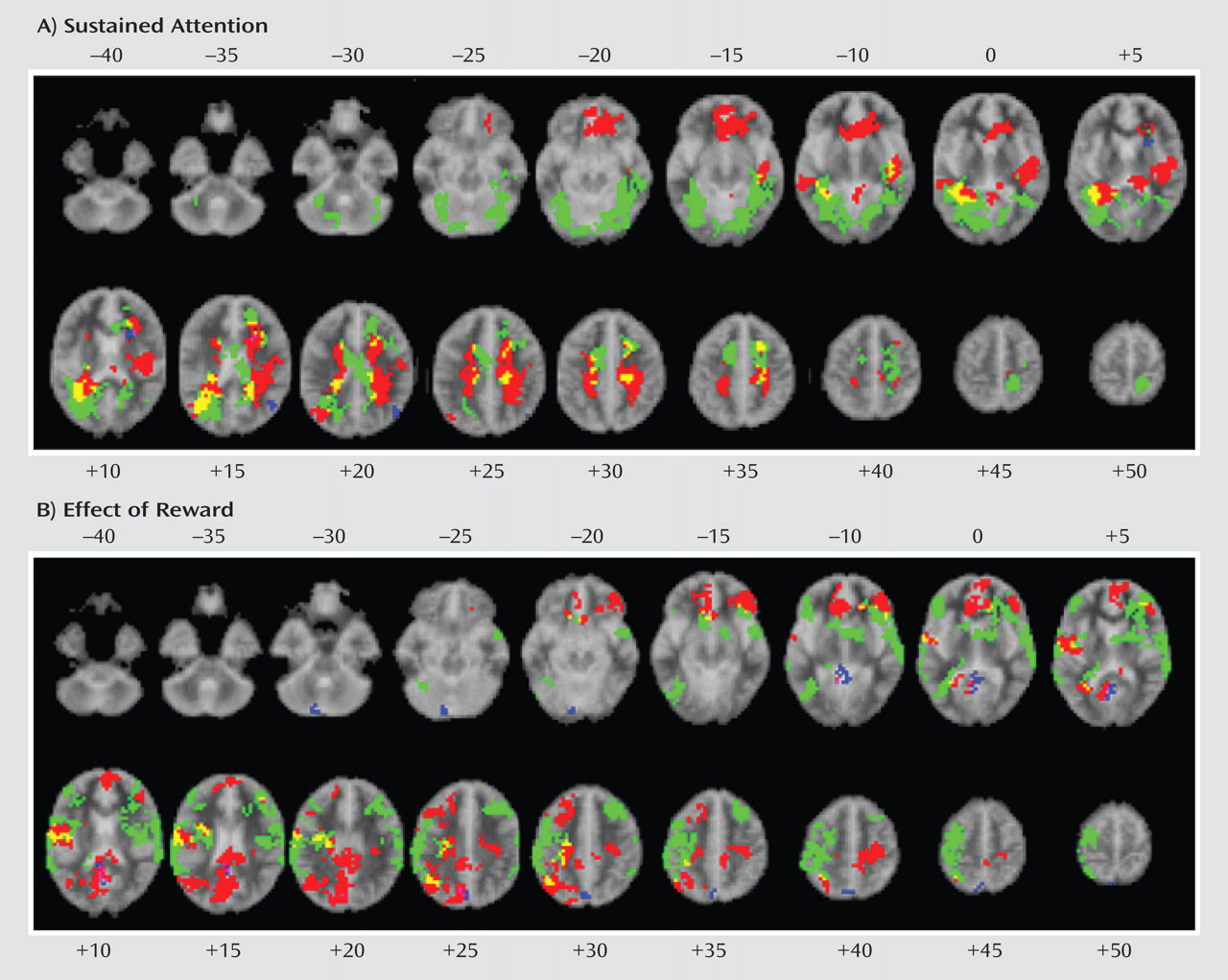
Figure 1 ).
In order to measure effects of sustained attention, fMRI was used to contrast brain activation to nonrewarded target trials with brain activation to nontarget trials. The number of nonrewarded target trials was subtracted from the number of rewarded target trials in order to measure the effect of reward.
Analysis of Performance Data
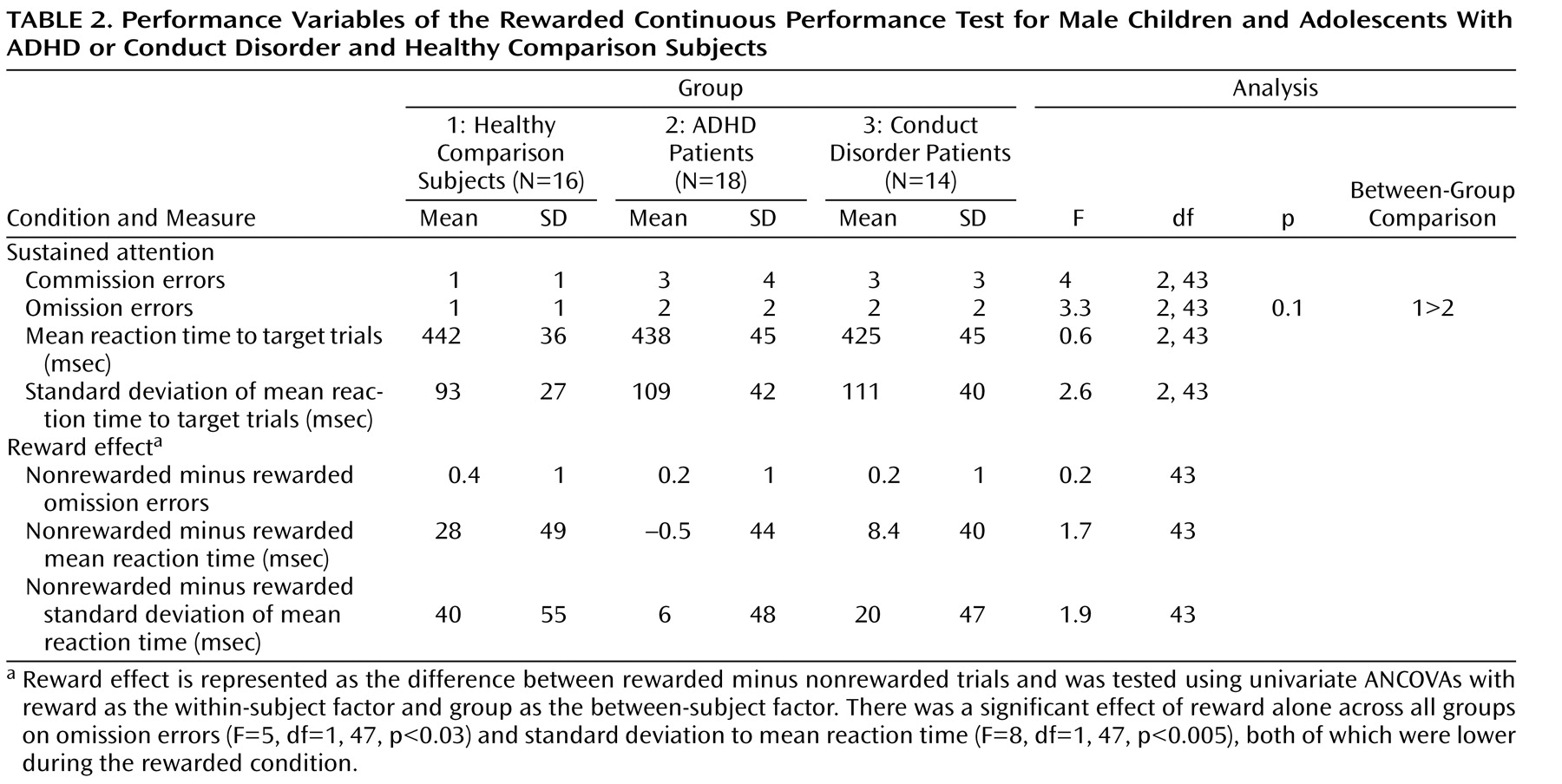
Although there were no significant differences in IQ or age, ADHD patients had slightly lower IQ scores relative to conduct disorder patients and healthy comparison subjects, and conduct disorder patients were, on average, 1 year younger than ADHD patients and healthy comparison subjects. Consequently, multiple univariate analyses of covariance (ANCOVAs), with IQ and age as covariates, were conducted to compare the following task performances between the three groups: omission errors to targets, commission errors (response to nontargets), mean reaction time to target trials, and intrasubject response variability of reaction time to targets (standard deviation). The reward effect was tested using univariate-repeated ANCOVAs, with reward as the within-subject factor and group as the between-subject factor. The false discovery rate was used to adjust p values for multiple testing.
fMRI Acquisition
Gradient-echo echo planar magnetic resonance imaging (MRI) data were acquired via the GE Signa 1.5T Horizon LX System using a semiautomated image quality control procedure. A quadrature birdcage head coil was used for radio frequency transmission and reception. In each of 16 noncontiguous planes parallel to the anterior-posterior commissural, 208 T2-weighted magnetic images, which depicted blood-oxygen-level-dependent (BOLD) contrast covering the whole brain, were acquired (TE=40 msec, time to repeat=1.8 sec, flip angle=90°, in-plane resolution=3.1 mm, slice thickness=7 mm, slice skip=0.7 mm).
fMRI Analysis
Time series analysis for each subject was established according to a previously published wavelet-based data resampling method for assessing fMRI data
(27,
28) . Using rigid body and affine transformation, individual maps were registered to Talairach standard space
(29) . A group brain activation map was then produced for each experimental condition, and hypothesis testing was conducted at the cluster level. In essence, a voxel-wise test at p<0.05 was conducted to identify any voxels that might be activated by a subsequent test at a cluster level threshold of p<0.01 to remove false positive clusters produced by the voxel-level test. This combination (voxel/cluster) of tests, coupled with permutation testing, allows for excellent type I error control at the cluster level
(27,
28) . For each task, we expected less than one false positive activated cluster at a p value <0.05 at the voxel level and <0.01 at the cluster level. For between-group comparisons, one-way ANCOVAs, with group as a factor and age and IQ as covariates, were conducted using a randomized test to assess voxel- or cluster-wise differences
(27,
28) . For ANCOVAs, less than one false positive activated cluster was expected at a p value <0.05 for both voxel and cluster comparisons. In each significant cluster of the ANCOVAs, statistical measures of BOLD response for each participant were extracted, and post hoc least significance difference t tests (corrected for multiple comparisons) were conducted to identify between-group differences.
In large connected clusters, we identified local maxima that were farther apart than the upper bound of the likely Talairach mapping error (3 voxel radius:10 mm)
(30) . Voxels were then assigned to the nearest local maximum with a statistic value that exceeded that of the voxels.
Discussion
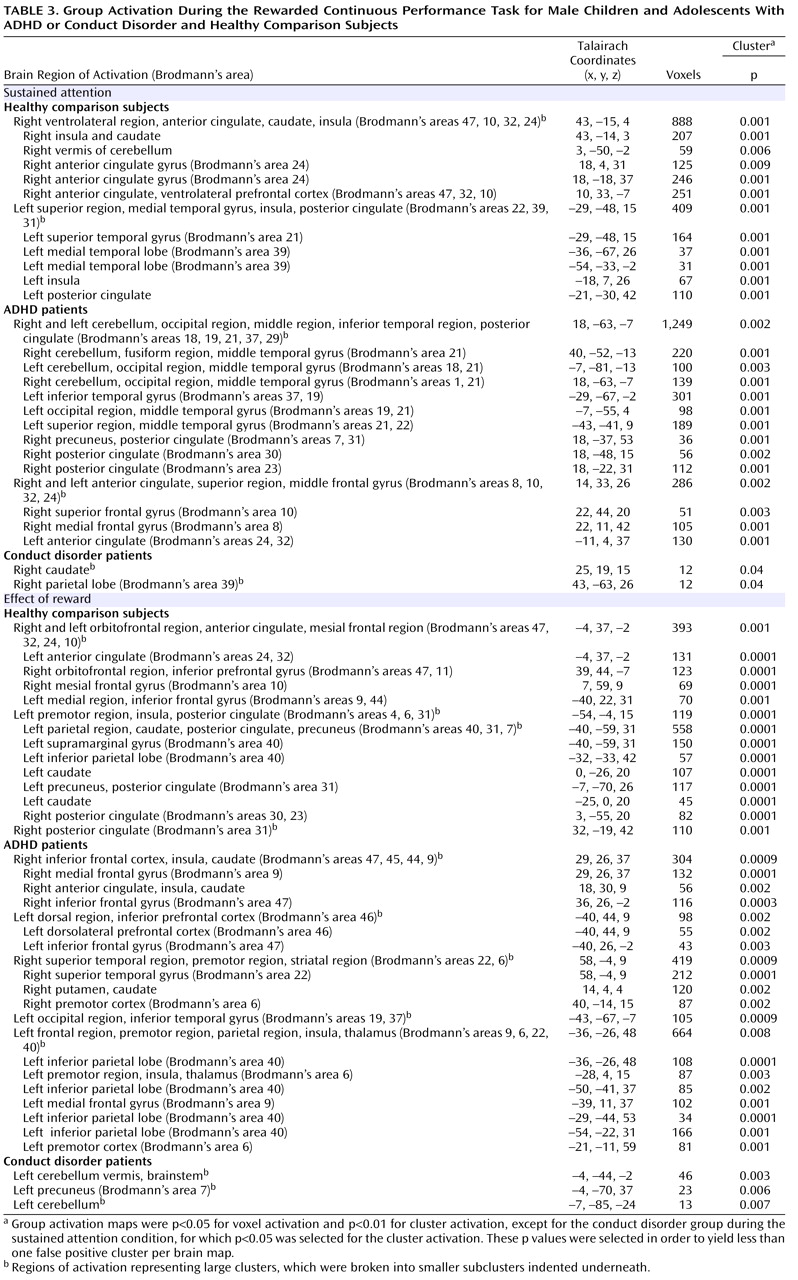
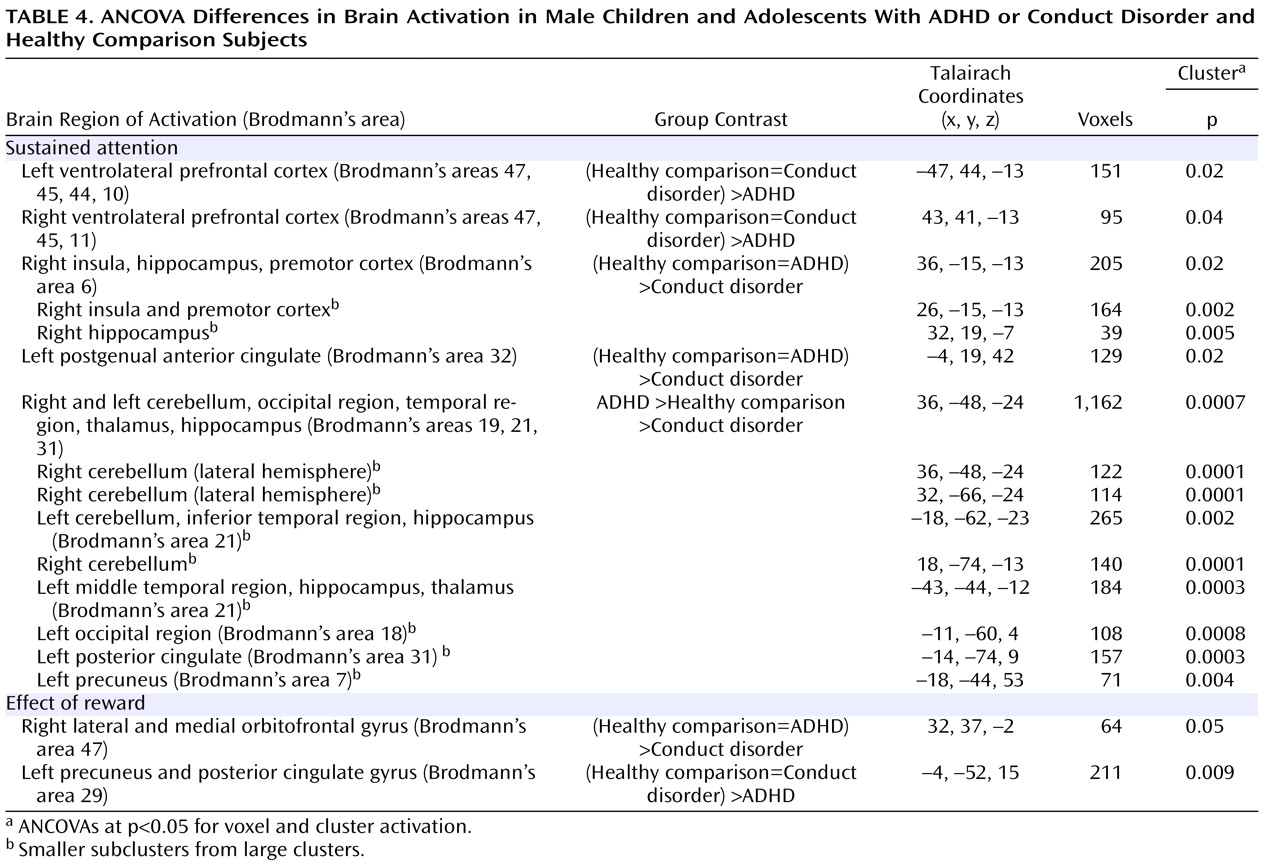
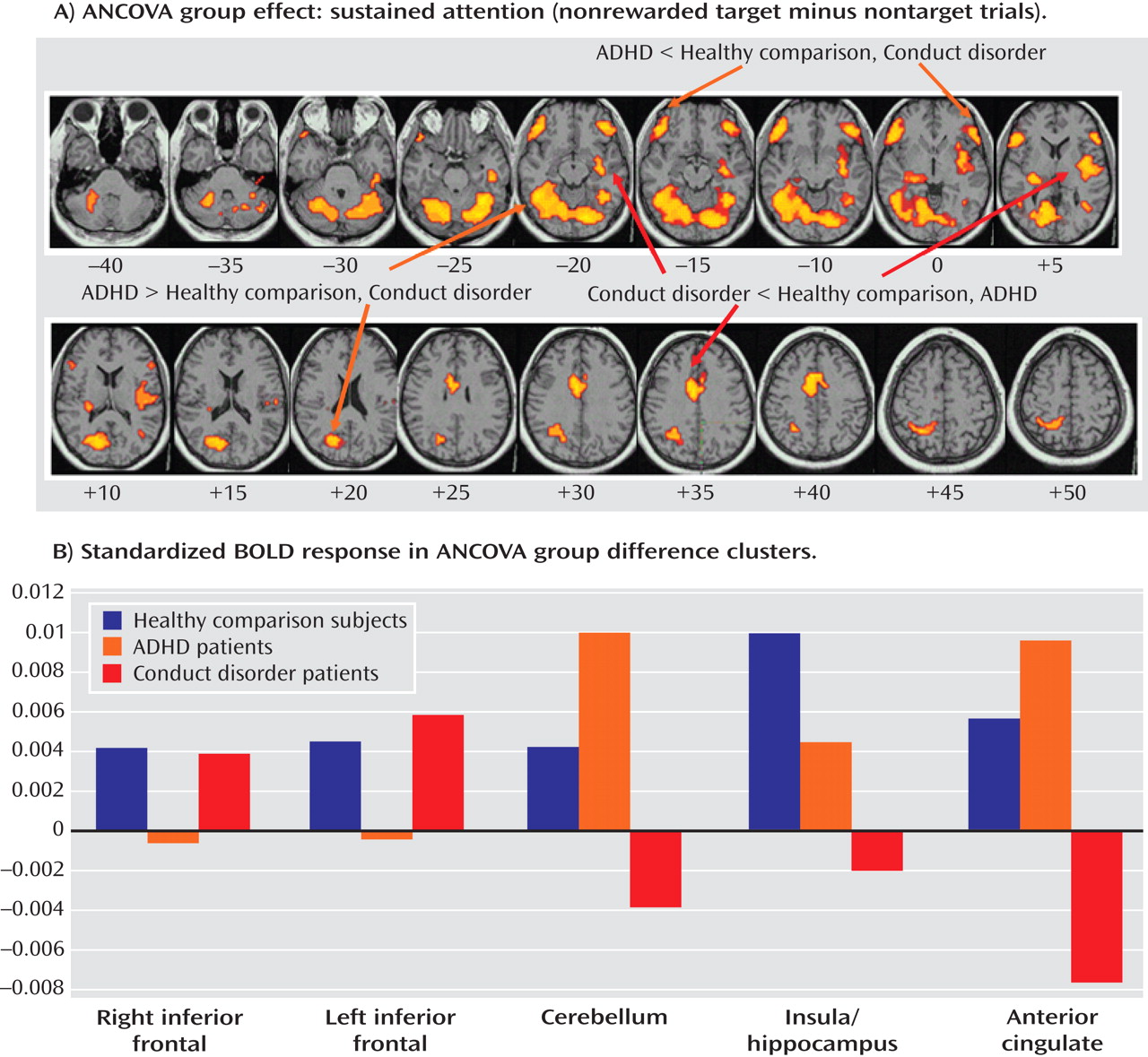
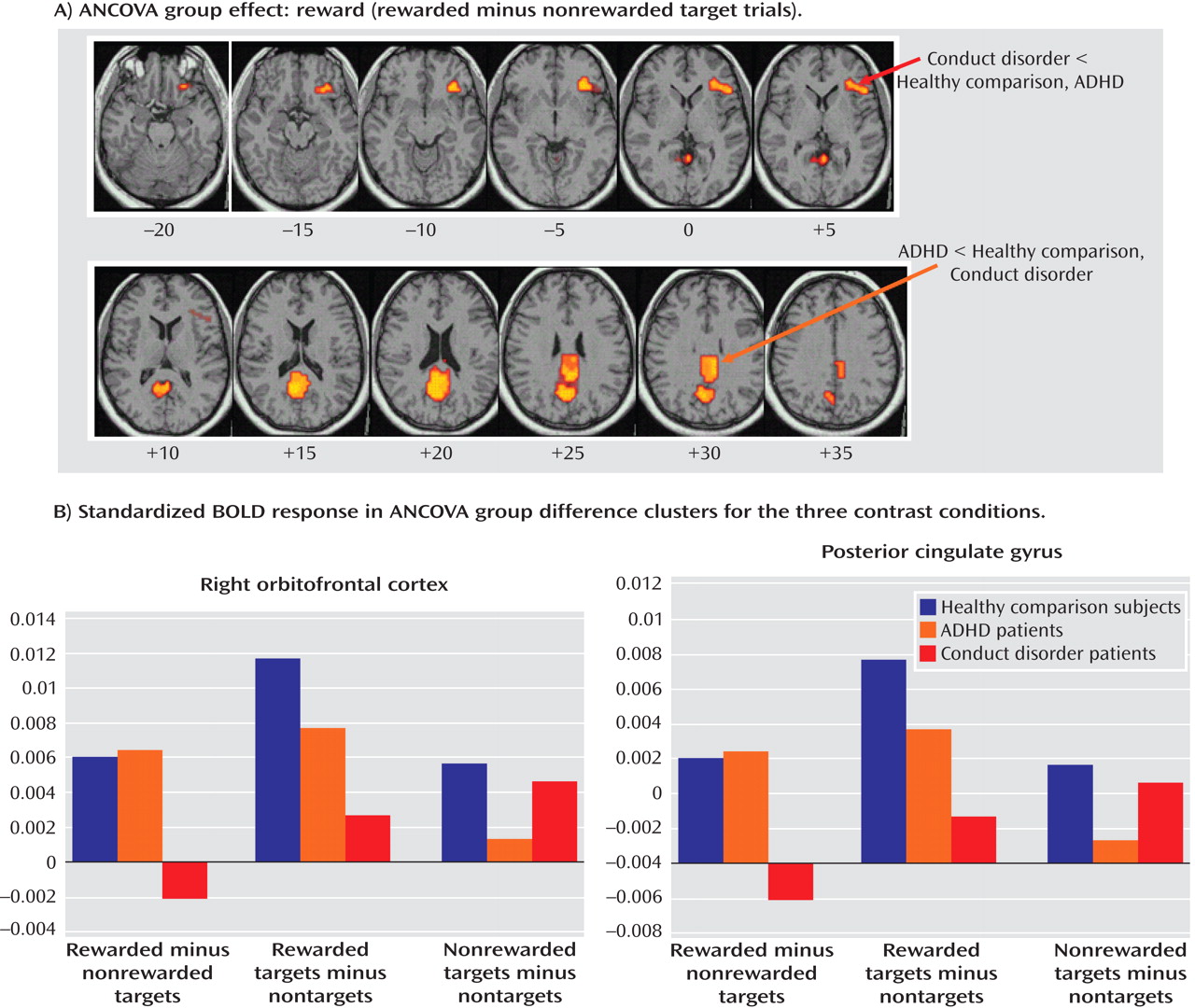
Patients with noncomorbid conduct disorder and noncomorbid ADHD showed disorder-specific brain abnormalities during the two task conditions. During sustained attention, conduct disorder patients demonstrated disorder-specific dysfunction in paralimbic regions of the insula and hippocampus and the postgenual anterior cingulate gyrus. However, ADHD patients demonstrated disorder-specific underactivation in the right and left ventrolateral prefrontal cortex but increased activation in a cluster comprising the cerebellum, thalamus, and hippocampus. During the reward condition, conduct disorder patients showed disorder-specific dysfunction in the right orbitofrontal cortex, while ADHD patients showed disorder-specific reduced brain activation in the posterior cingulate and precuneus.
The underactivation in conduct disorder patients during sustained attention was in regions of the paralimbic system that lie at the interface between emotion and cognition. The postgenual anterior cingulate is connected to frontal-parietal attentional networks but is crucial for motivation and arousal
(31,
32) . A more anterior part of the insula has been shown to contribute to sustained attention
(33), and the hippocampus plays a role in selective visual attention to targets
(34) . These findings suggest that conduct disorder is characterized by reduced brain activation in paralimbic regions that contribute to attention networks, presumably through their role in motivation.
The dorsal anterior cingulate gyrus has previously been found to be dysfunctional in boys with conduct disorder during the presentation of images with negative emotional valence
(15) . Other limbic regions, such as the amygdala and its connectivity to the orbitofrontal cortex, have been found to be dysfunctional in callous-unemotional children with conduct disorder and/or oppositional defiant disorder relative to children with ADHD and comparison subjects during fearful face processing
(16) . Structural studies have found abnormalities in orbitofrontal and temporal regions, including the amygdala and hippocampus, in boys with conduct disorder
(35,
36) . The findings of functional abnormalities in limbic structures during attention are thus consistent with the hypothesis that aggressive behavior is related to abnormalities in the paralimbic system, which mediates the control of motivation that may effect attention functions
(14) .
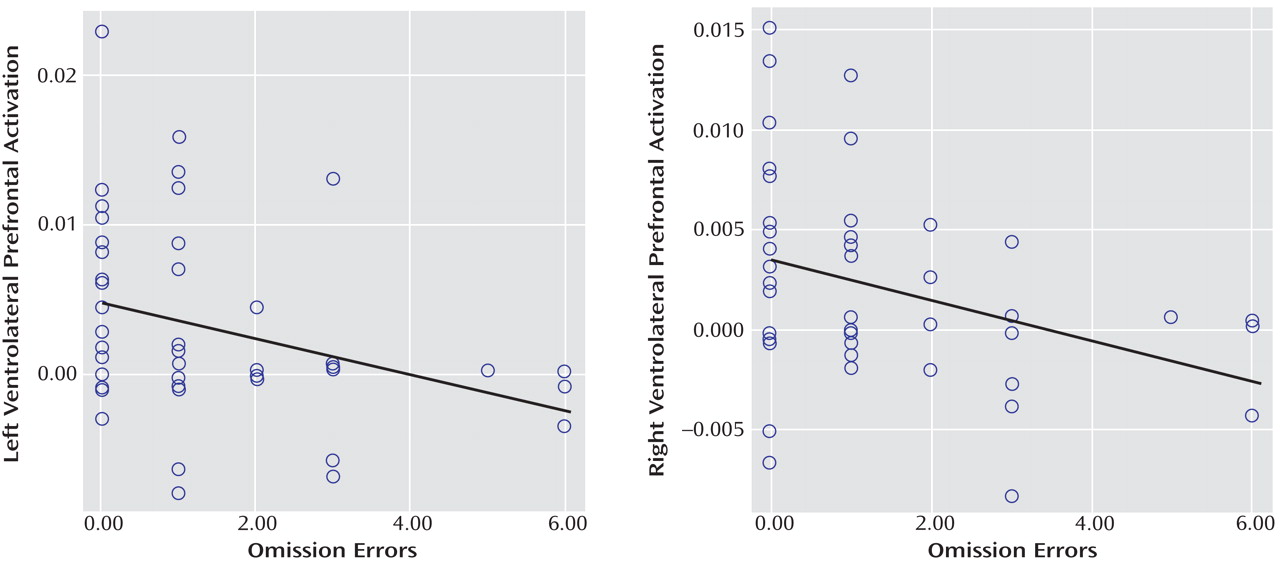
The underactivation in ventrolateral prefrontal brain regions during sustained attention in ADHD patients was hypothesized. Lateral inferior and orbital prefrontal abnormalities have consistently been observed in boys with ADHD during a range of cognitive functions, including inhibition
(4,
5,
17) and attention tasks during fMRI
(6), and in adolescent girls during an auditory Continuous Performance Test
(7) . Left and right ventrolateral prefrontal activation was correlated across all subjects with omission errors, which were reduced at a tendency level in ADHD patients, suggesting that the underactivation in these regions may cause poor sustained attention capacity. The finding of disorder specificity of ventrolateral prefrontal underactivation is consistent with our previous findings of disorder-specific underactivation of left ventrolateral prefrontal activation in the same ADHD patient group relative to healthy comparison subjects and conduct disorder patients during a motor response inhibition task
(17) . To our knowledge, this provides the first evidence that the consistently reported ventrolateral prefrontal dysfunction in ADHD patients during tasks of attention and inhibition may be specific to the disorder, at least when compared with conduct disorder.
The increased activation in posterior and subcortical regions in ADHD patients could have been a compensation for the poor frontal activation. The cerebellum, hippocampus, and thalamus are activated during the performance of sustained attention tasks
(9,
33,
37), and a prefrontal dysfunction in ADHD patients may have prompted the recruitment of posterior regions of a fronto-striato-thalamo-cerebellar attention network. The findings of cerebellar abnormalities in ADHD patients during attention are relatively novel in the context of functional imaging but are consistent with robust evidence for volumetric abnormalities of this brain area in structural imaging studies
(38) . A possible alternative interpretation could be that ADHD patients showed lower activation in the cerebellum during nontarget trials, and thus the activation difference between target and nontarget trials in this region was enhanced in these patients. Interestingly, this cluster was reduced in patients with conduct disorder relative to healthy comparison subjects, suggesting that this region may be another area of neurofunctional differentiation between ADHD and conduct disorder.
The findings of underfunctioning of paralimbic contributions to attentional networks in conduct disorder patients and of lateral fronto-cerebellar network abnormalities in ADHD patients support the theory, based on neuropsychological studies, that motivational abnormalities mediated by the paralimbic system may be underlying conduct disorder, while attentional cognitive abnormalities mediated by fronto-striato-cerebellar networks may be underlying ADHD
(4,
5,
14,
17) . This is further supported by the findings in the present study of a process-dependent dissociation of prefrontal dysfunction in both patient groups, which was in a bilateral ventrolateral prefrontal location during sustained attention in ADHD patients and a more medial right hemispheric orbitofrontal location in conduct disorder patients.
Although the ventrolateral prefrontal cortex has been reported to be involved in sustained attention
(33,
37), more medial regions of the orbitofrontal cortex are known to play a role in motivation and monetary reward
(39,
40) . The orbitofrontal cortex is also known to modulate paralimbic regions that mediate aggression
(14) . The orbitofrontal cortex, together with temporal and limbic areas, was reduced in gray matter in boys with conduct disorder
(35) . It has been hypothesized that abnormalities in reward computations that are mediated by the orbitofrontal cortex, leading to enhanced frustration, could trigger reactive aggression, which would explain the link between aggression, abnormalities in reward-related brain regions, and orbitofrontal abnormalities
(14) . Our findings are consistent with this theory and, to our knowledge, provide the first functional evidence that aggressive and antisocial behaviors are related to a dysfunction of the orbitofrontal cortex and paralimbic areas during reward and attention.
The findings of underactivation in reward-related orbitofrontal regions in patients with conduct disorder only support the hypothesis of a disorder-specific hyposensitivity to reward in conduct disorder. This is consistent with findings of reduced autonomic responses in children with conduct disorder relative to children with ADHD and healthy comparison subjects during emotional stimuli
(41) . Our findings do not support the theory of a hypersensitivity to reward in ADHD patients. To the contrary, our findings show reduced recruitment of the posterior cingulate and precuneus that are important for visual-spatial attention allocation to visually or motivationally salient events, such as reward
(42) . This is consistent with evidence of a dopamine deficiency in ADHD patients
(43,
44), which is associated with a blunted attentional response to saliency
(45) . We previously found reduced posterior cingulate and precuneus activation in boys with ADHD during motor timing
(4), error detection
(5,
17), and attention allocation
(46), which correlated with scores of hyperactivity on the Strength and Difficulties Questionnaire
(5) . The posterior cingulate therefore appears to be an important—but thus far neglected—brain region of dysfunction in ADHD patients that could be the neural substrate for the reduced capacity in these patients for attention allocation to relevant targets.
In the present study, the three groups did not differ in performance, although there was a tendency for more omission errors among ADHD patients relative to healthy comparison subjects. This finding is not consistent with the majority of studies that reported deficits in children with ADHD during the continuous performance task
(2,
3), although it is consistent with findings from several studies on conduct disorder
(11) . ADHD adolescents are usually less impaired than ADHD children, and our fMRI adaptation of the Continuous Performance Test using single targets—designed to reduce working memory demands and confounds of performance differences between groups—was deliberately easier than more commonly used double-target versions (i.e., Continuous Performance Test [A–X]).
One common problem with fMRI adaptations of continuous performance tasks is that motor responses to targets are not controlled, since a motor response to nontargets would introduce unwanted attentional demands. Thus, some activation differences could have been potentially motor- rather than purely attention-related. However, the main ANCOVA findings were not in motor regions. Inferior prefrontal activation, which was reduced in ADHD patients, is known to mediate sustaining attention during motor-controlled vigilance and parametric-sustained attention tasks
(33,
37), and this activation correlated with omission errors, which were the main attention measure of the task. Likewise, the paralimbic areas that were reduced in conduct disorder patients were not located in motor regions.
The careful selection of patients who differed from each other in conduct and ADHD problems is a strength of the present study. However, this selection limits generalizability to more commonly occurring comorbid cases. Future research should clarify the extent to which comorbidity results from the blending of conduct disorder and ADHD.
The findings of the present study suggest that conduct disorder and ADHD, two clinically overlapping disruptive disorders, may be based on different etiopathophysiological substrates implicating orbitofrontal-limbic motivational networks in patients with conduct disorder and fronto-cerebellar attention networks in patients with ADHD. Our results can be considered a first step toward the delineation of the specific biomarkers of the two diagnostic disorders, which will ultimately contribute to the development of more objective phenomenological differentiation and disorder-specific tailored treatment.