Theoretical and empirical evidence suggest that the cerebellum plays a role in the mechanisms and symptoms underlying schizophrenia. Evidence of cerebellar involvement in perceptual timing, in addition to the role of the cerebellum in motor coordination, has prompted speculation that it is a dedicated system for representing time in psychological as well as motor domains
(1) . The cognitive dysmetria model of schizophrenia
(2,
3) posits that some symptoms of the disorder may arise from abnormal temporal coordination of neural processes underlying cognition. Empirical support for this model derives from studies suggesting that cerebellar structure and function are abnormal in individuals with schizophrenia
(4,
5) and that cerebellar abnormalities are associated with poor long-term outcome
(6) and greater cognitive dysfunction
(7) among patients with the disorder.
It is unlikely that a single mechanism can explain the diverse symptoms of schizophrenia. Instead, different symptom domains probably possess unique targets for treatment. Hence, recent research has focused on targeted treatments for various cognitive symptoms of the disorder. For example, early pharmacological data suggest that nicotinic alpha 7 subunit selective cholinergic agents may improve attentional deficits
(8) and muscarinic M
1 receptor agents may improve verbal learning and short-term memory
(9) . However, little is known about potential therapeutic targets in the cerebellum.
One of the best ways to assess cerebellar function is via eye-blink conditioning, wherein a conditioned stimulus, such as a tone, is paired with an unconditioned stimulus, such as an air puff to the eye. The unconditioned stimulus air puff evokes a reflexive eye-blink, which is the unconditioned response. When the conditioned stimulus is repeatedly paired with the unconditioned stimulus, a conditioned response (an eye-blink) develops prior to the onset of the unconditioned stimulus air puff. This conditioned response requires temporal precision and is therefore an ideal test of the integrity of cerebellar timing mechanisms. Importantly, prominent abnormalities during this task have been found in individuals with schizophrenia
(13 –
17) . Moreover, eye-blink conditioning responses may be mediated by neurotransmitter systems on which secretin acts.
Although secretin, a hormonal agonist for the prototype group B G-protein-coupled receptors, was the first recognized human hormone and its gastrointestinal functions have been widely studied, relatively little is known about its effects and mechanisms of action in the CNS. Animal models indicate that secretin is endogenously released in the cerebellum
(10,
11,
18) . Yung et al.
(10) showed that secretin is present in the soma and dendrites of Purkinje cells and secretin receptors are present on basket and Purkinje cells in the cerebellar cortex. Moreover, electrophysiological evidence has demonstrated that secretin increases the amplitude of spontaneous and evoked inhibitory postsynaptic currents that originate from interneurons and are recorded from Purkinje cells and decreases the paired-pulse ratio of the evoked inhibitory postsynaptic currents. Accordingly, secretin appears to increase the firing of presynaptic neurons, which then inhibit the Purkinje cells by selectively facilitating inhibitory gamma-aminobutyric acid (GABA)-ergic inputs via a presynaptic and cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent mechanism. These findings suggest that secretin is synthesized in Purkinje cells and then released following depolarization to promote recurrent inhibition, which stabilizes these cells via facilitated GABA-ergic afferent activity originating from presynaptic basket cells. Secretin’s mechanism of action appears to be similar to a recently discovered endocannabinoid-mediated retrograde signaling mechanism termed depolarization-induced suppression of inhibition
(19) . However, in the case of secretin, depolarization of Purkinje cells increases, rather than decreases, inhibitory input to the Purkinje cells. Taken together, this evidence constitutes a strong argument for a neuromodulatory role of secretin in the cerebellum. Additionally, given the evidence of secretin’s effects in cerebellar cellular function and its proposed mechanism of action on Purkinje cells, eye-blink conditioning methodology is well suited for examining the CNS effects of secretin in humans. This objective is an important one, since secretin has not been demonstrated to influence human CNS function, although other members of the same peptide family act as neuropeptides
(10) . The centrality of the cerebellum in acquiring eye-blink responses has been demonstrated in both nonhuman
(20 –
24) and human studies
(25 –
27) . Although the hippocampus, amygdala, and frontal regions may modulate conditioned response acquisition and performance, cerebellar lesions alone prevent learning of conditioned responses and abolish previously learned responses
(28) . Moreover, eye-blink conditioning magnitude correlates with cerebellar volume
(26) . The cerebellar cortex acts on the deep cerebellar nuclei—the only source of cerebellar output—exclusively through the inhibitory effects of Purkinje cells on these nuclei. Consequently, populations of Purkinje cells are thought to directly affect conditioned response rates by facilitating or depressing output of the deep nuclei. Given evidence that secretin inhibits Purkinje cells by facilitating GABA-ergic effects of basket cells on Purkinje cells
(10), we predicted that secretin administration would enhance eye-blink conditioning. Specifically, secretin should act to inhibit Purkinje cells, thus releasing their inhibition (i.e., increasing the gain) on the conditioned response generating deep nuclei. Abnormalities in the acquisition and timing of eye-blink conditioning have been widely reported in studies of schizophrenia
(13 –
17) . Although studies of delay eye-blink conditioning with small sample sizes of schizophrenia patients have reported no differences in the percentage of conditioned responses
(15) or facilitation
(16,
17), a recent study in our laboratory (unpublished data available upon request from Bolbecker AR et al.) found impaired conditioning in a large sample of schizophrenia patients (N=62), replicating previous studies of eye-blink conditioning in schizophrenia
(13,
14) . Importantly, repeated eye-blink conditioning training across multiple independent sessions does not appear to significantly improve eye-blink conditioning in patients with schizophrenia, pointing to the refractory nature of the cerebellar-mediated learning deficit in the disorder. Previous research indicates specific roles for secretin and its receptors in the inhibition of cerebellar Purkinje cells
(10) . This intriguing finding, combined with extensive knowledge of cerebellar mechanisms underlying eye-blink conditioning, led to the important prediction that secretin administration would increase conditioned eye-blink responses, which would be clearly demonstrated in populations such as schizophrenia patients, in whom profound eye-blink conditioning deficits have been reported. In the present study, a double-blind, placebo-controlled, between-subjects design indicated that subcutaneous administration of secretin significantly increased eye-blink conditioning in individuals diagnosed with schizophrenia.
Results
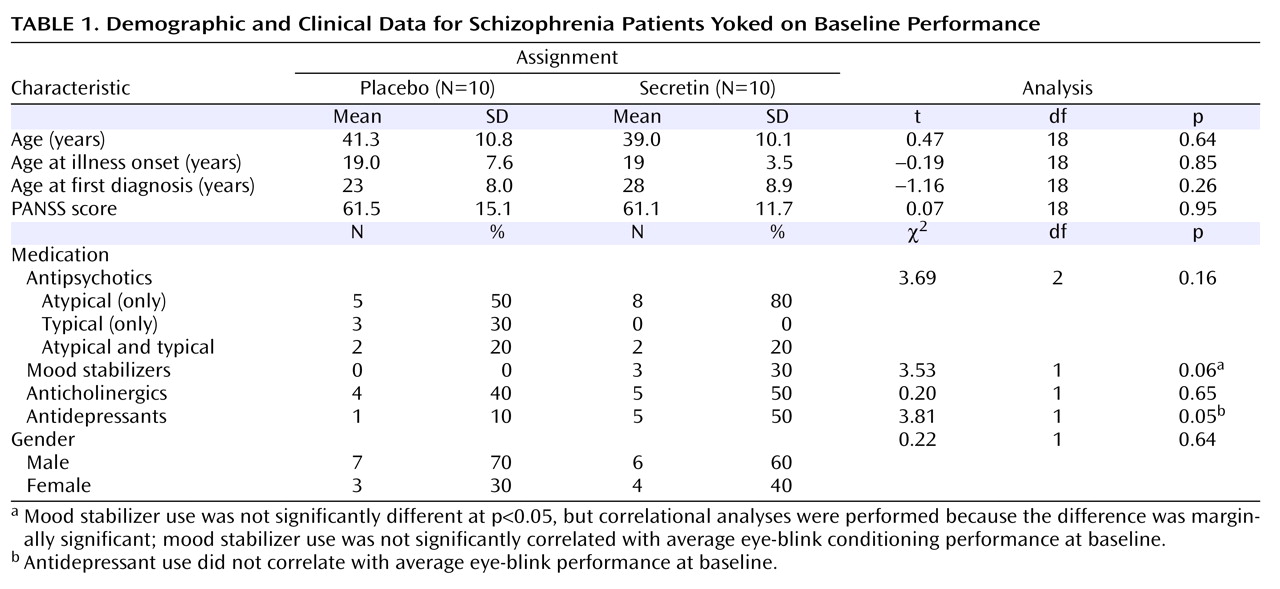
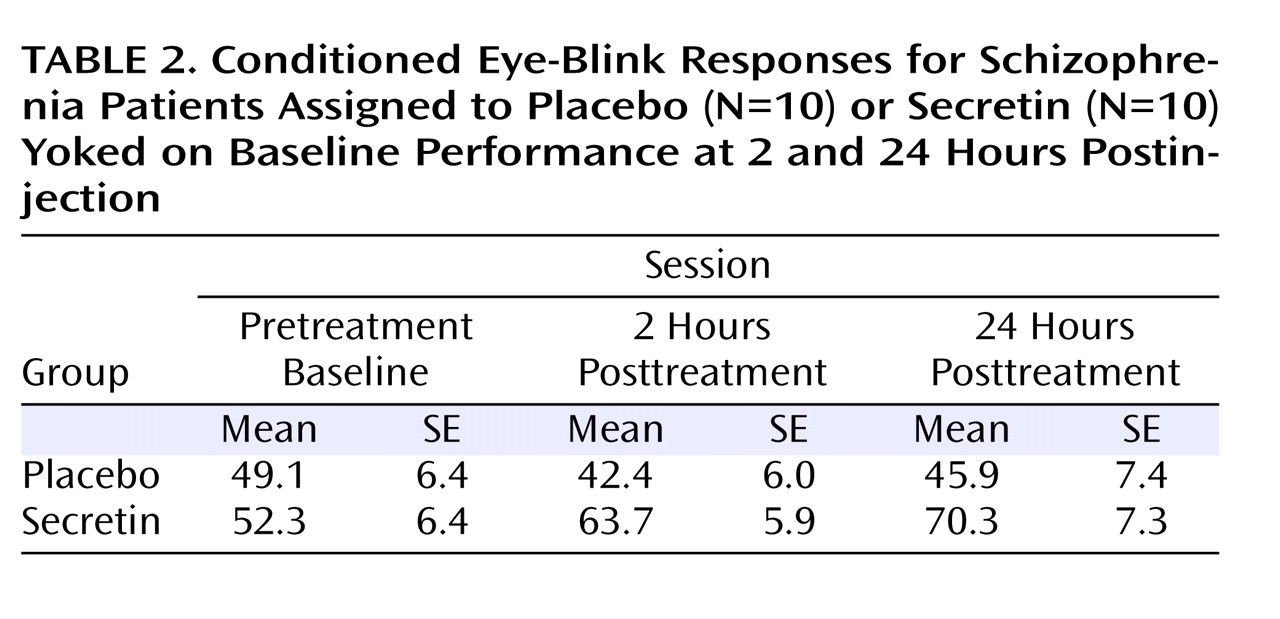
Table 2 shows the average percentage of conditioned eye-blink responses for each session. As expected, given the baseline yoking procedure, statistical analyses indicated that the overall mean percentage of conditioned eye-blink responses at pretreatment baseline did not differ between treatment groups (F=0.12, df=1, p=0.73; η
P 2 =0.01). Additionally, there was no main effect of block, nor was there a block-by-treatment group interaction. However, at 2 hours posttreatment, overall conditioning in the secretin group was significantly higher compared with the saline group (F=6.4, df=1, p=0.02; η
P 2 =0.27). Neither the main effect of block nor the block-by-treatment group interaction was significant. At 24 hours posttreatment, overall conditioning in the secretin group remained significantly higher (F=5.5, df=1, p=0.03; η
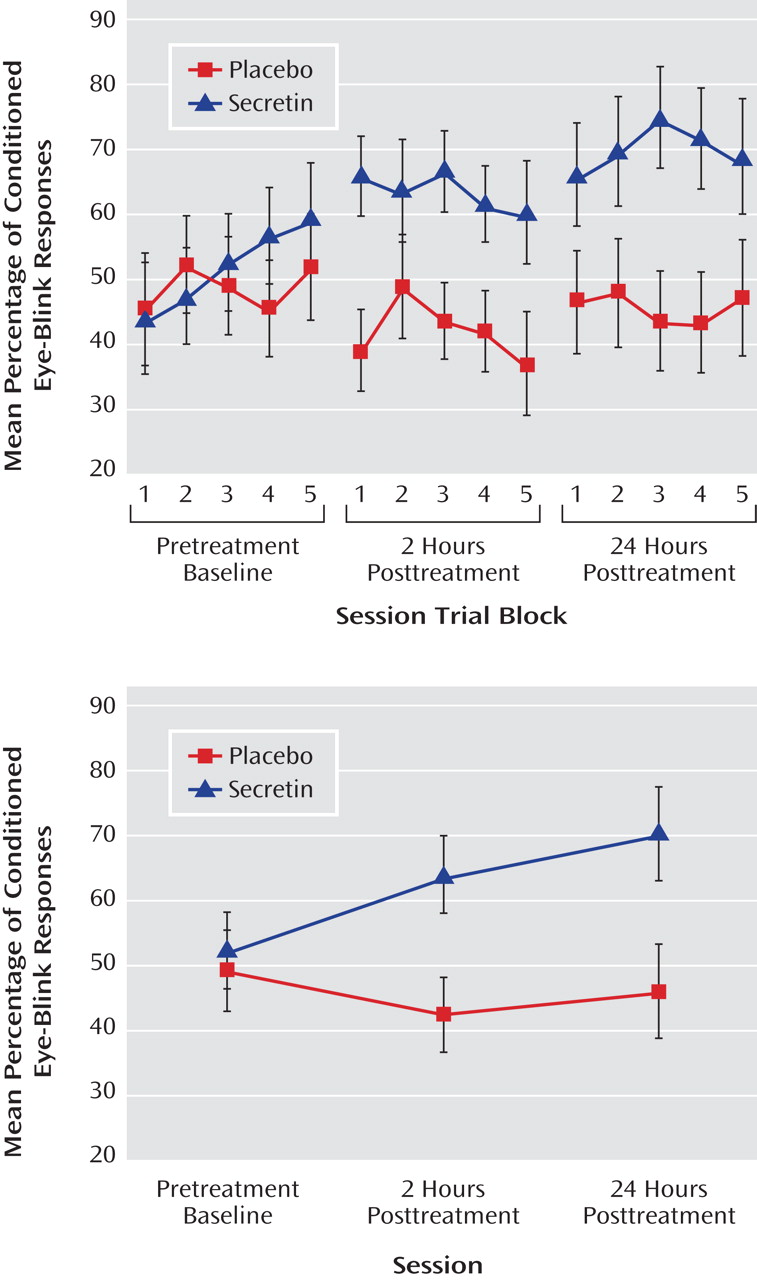
P 2 =0.25). To examine the effect of treatment across sessions (pretreatment baseline, 2 hours posttreatment, and 24 hours posttreatment), the mean percentage of conditioned eye-blink responses was computed for each subject within each of the three sessions. Secretin administration resulted in higher levels of conditioning at 2 and 24 hours posttreatment compared with levels during the pretreatment baseline recording session, whereas conditioning in the saline-treated group remained relatively unchanged (
Figure 1 ). Statistical analyses revealed a main effect of session (F=5.7, df=1.9, p=0.01; η
P 2 =0.25), and, importantly, a session-by-treatment group interaction (F=3.5, df=1.9, p=0.04; η
P 2 =0.17). Although the main effect of treatment only approached statistical significance (F=3.9, df=1, p=0.06), it was associated with a large effect size estimate (η
P 2 =0.17)
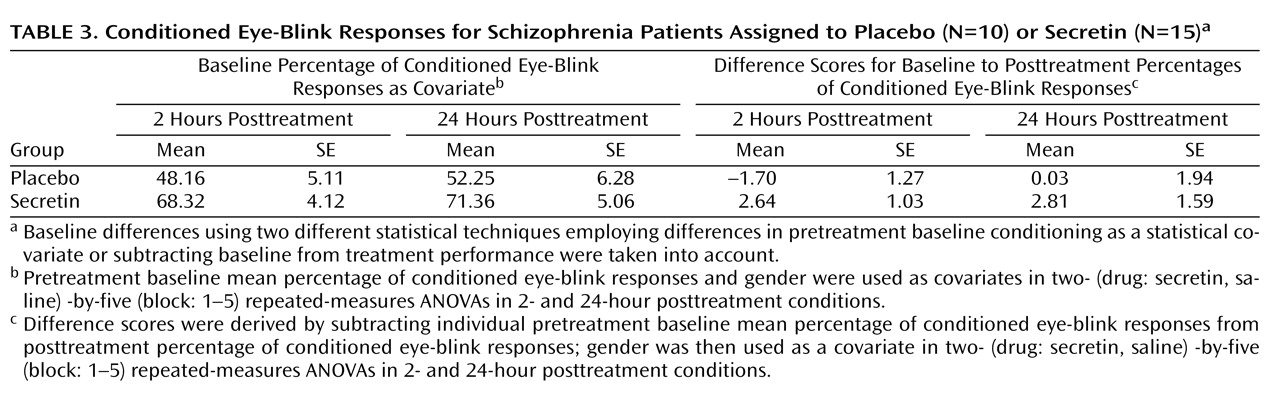
(32) . In the secretin group, the mean increase in conditioning from baseline to 2 hours posttreatment (mean=10.3% [SE=5.5%]) approached significance (p=0.10) and became statistically significant (p=0.03) at 24 hours posttreatment (mean=14.7% [SE=5.4%]). In the saline group, mean conditioning differences between baseline and 2 (mean=5.7% [SE=5.4%]) and 24 (mean=3.4% [SE=5.4%]) hours posttreatment were not statistically different. Importantly, analysis of the entire nonyoked sample (placebo group: N=10; secretin group: N=15) produced the same pattern of results. Within each posttreatment session, including the mean percentage of conditioned eye-blink responses at pretreatment baseline as a covariate produced significant group differences at 2 (p=0.007) and 24 hours (p=0.03) posttreatment. When preinjection baseline percentage of conditioned eye-blink response scores were subtracted from postinjection percentage of conditioned eye-blink responses, significant group differences were observed at 2 hours posttreatment (p=0.007) but not 24 hours posttreatment (p=0.28). The similarity of mean percentage of conditioned eye-blink responses at 2 and 24 hours posttreatment suggests that this is likely attributable to increased variance at 24 hours posttreatment (
Table 3 ). In two separate two- (drug: secretin, saline) -by-two (session: 2 hours posttreatment, 24 hours posttreatment) repeated-measures ANOVAs, a main effect of group was observed both when the individual baseline percentage of conditioned eye-blink response average was used as a covariate (p=0.006) and when the pretreatment baseline percentage of conditioned eye-blink response scores were subtracted from the posttreatment percentage of conditioned eye-blink responses at 2 and 24 hours posttreatment (p=0.04). The consistency of group differences, both within and across sessions, using these different methods of analysis makes a compelling argument that there were true differences between the groups.
Discussion
Our finding that subcutaneous secretin administration increased the rate of conditioned responses in a well-established associative learning task among individuals diagnosed with schizophrenia is important for two reasons. First, to the best of our knowledge, these results provide the first behavioral evidence that secretin affects the function of the human CNS. Moreover, the observed protherapeutic effects of secretin on this cerebellar-dependent measure of conditioning are consistent with the putative role of secretin in the cerebellum, as established in a rodent model
(10,
11,
18) . Second, the fact that these improvements in cerebellar-dependent conditioning were observed in individuals with schizophrenia, a psychiatric disorder previously shown to have eye-blink conditioning deficits
(13,
14), is important because—taken together with the animal model
(10,
11,
18) —the results suggest a mechanism for these deficits. The apparent beneficial effects of secretin administration on conditioning rate in our study are especially noteworthy given that 1) a majority of the patients in the secretin group had been pre-exposed to the eye-blink conditioning task but exhibited no practice effects and 2) patients in the saline-treated group exhibited no evidence of increased rates of conditioning, even after exposure to 270 conditioning trials in a span of 24 hours. In other words, secretin’s effects were noteworthy given the apparent refractory nature of this cerebellar-mediated conditioning deficit in schizophrenia patients. The fact that secretin was shown to affect cerebellar-dependent learning in schizophrenia is significant given compelling evidence suggesting that the cerebellum plays a role in a wide variety of psychological functions, including cognitive and affective processes
(33,
34) . As detailed in a review by Schmahmann
(34), reciprocal topographic projections connect the cerebellum to brain areas implicated in schizophrenia, such as the thalamus, limbic system, motor region, prefrontal region, and posterior parietal cortex. Hence, it has been suggested that the cerebellum integrates and coordinates information from different functional domains
(2,
33,
34) . Cerebellar abnormalities have been reported in schizophrenia
(5,
35), including decreased cerebellar size
(7,
36,
37) . Interestingly, cerebellar volume deficits have been shown to be reliable indicators of poor long-term outcome
(6) . Moreover, observed cerebellar volume deficits are correlated with greater cognitive dysfunction
(7), lending support to the theory that cerebellar dysfunction contributes to cognitive dysmetria
(2,
3) . The role of the cerebellum in timing suggests that its overarching role may be to integrate and coordinate neural signals in time. Thus, cerebellar abnormalities in schizophrenia may lead to discoordination of temporal information that gives rise to symptoms of schizophrenia. Taken in the context of the present finding that secretin improved eye-blink conditioning performance in individuals with schizophrenia during a cerebellar-dependent task, this neuropeptide system may prove useful as a therapeutic target for improving the cerebellar components of cognition by remediating timing deficits in schizophrenia.
Although the present results are novel and theoretically interesting, there are several experimental limitations to the study that warrant follow-up in subsequent research. First, although we attempted to control for baseline differences in conditioning by yoking subjects on the basis of baseline performance, the source of these differences and their effects on the results are unclear. Additionally, the yoking procedure resulted in the loss of subjects in these analyses. Nevertheless, the same pattern of results was seen for the full sample when alternative statistical methods were employed in order to take baseline differences into account. Furthermore, there were performance differences between male and female subjects, limiting the interpretability of the analyses. However, these differences existed at baseline and persisted throughout the study, which suggests that they were not related to drug condition. Moreover, no sex effects on eye-blink conditioning were found in our earlier study
(13) or in our more recent, larger schizophrenia study (unpublished data available upon request from Bolbecker AR et al.). Hence, these differences may be an artifact of small sample size.
The present study was notable because it showed an increase in eye-blink conditioning rates in a group of medically stable schizophrenia patients following secretin administration. This finding is particularly striking, since this group had not shown an increase in conditioned responses even after repeated exposure to the cerebellar-mediated task. The present results add to the increasing evidence of a cerebellar contribution to the pathophysiology of schizophrenia and point to a possible role for secretin in modulating cerebellar-mediated classically conditioned learning.