Study of patient tissues that express the biological mechanisms responsible for the disease condition is a tool that has been invaluable to the rest of medicine to elucidate pathological mechanisms. In psychiatry, study with autopsied brains may provide us with cellular and molecular changes at the end stage of the disorder. However, many confounding factors, including long-term exposure to medications, substance abuse, and smoking, may limit conclusions. The biological system that can overcome this limitation is awaited.
Olfactory deficits have been studied extensively in patients with schizophrenia
(1) . Abnormalities in odor identification using the University of Pennsylvania Smell Identification Test (UPSIT) are observed in a majority of patients with schizophrenia, whereas less than 15% of the general population shows such deficits
(2) . Impairment of olfactory identification has also been proposed as a premorbid marker of transition to the onset of schizophrenia
(3) . First-degree relatives of schizophrenia patients show decreased performance on the UPSIT that is intermediate between that of schizophrenia patients and normal comparison subjects, indicating that abnormalities in odor identification may be indicative of a genetic predisposition to the disorder
(4,
5) . At the molecular and cellular level, alteration in markers indicating abnormalities in neuronal development and differentiation has been reported in the olfactory epithelium, the most peripheral part of the olfactory system, in schizophrenia patients compared with healthy comparison subjects
(6) .
In this issue of the
Journal, Turetsky and Moberg
(7) report the presence of an odor-specific hyposmia that may denote a disruption of role of the intracellular signaling molecule cyclic adenosine monophosphate (cAMP) in signal transduction in schizophrenia. They found that odor detection threshold sensitivity for the odorant lyral was diminished, whereas the sensitivity to citralva was not, reflecting that these two stimuli activate cAMP signaling differently. The finding of this study raises the possibility that the olfactory system will provide the tissue source needed for more comprehensive molecular cell biology of schizophrenia. This work has several significant strengths. First, by using olfactory detection threshold sensitivity (i.e., the ability to detect a weak odor concentration), instead of olfactory identification, the authors may have succeeded in depicting specific abnormalities associated with the peripheral components of the olfactory system, such as olfactory epithelium and olfactory bulb. A question that usually rises when studying olfactory deficits is the level of the olfactory system responsible for them. Dysfunction of peripheral components may reflect deficits in specific cellular mechanisms, as the authors propose in this article, whereas more complex odor identification may involve the more inaccessible central brain areas involved in other cognitive deficits in schizophrenia (see
Figure 1 ). Second, this study is outstanding in that the authors identified selective deficits in olfactory detection threshold sensitivity only to an odor that can activate a key intracellular signaling involving cAMP, which indicates a specific susceptibility of this signaling system in schizophrenia. A parallel deficit is also observed in the patients’ unaffected first-degree relatives, suggesting that this deficit may be genetically mediated. The present work also suggests the possibility that a simple clinical examination may uncover an etiology-associated deficit of intracellular signaling in schizophrenia. Such deficits could form the basis for objective and quantitative biological markers for clinical psychiatry.
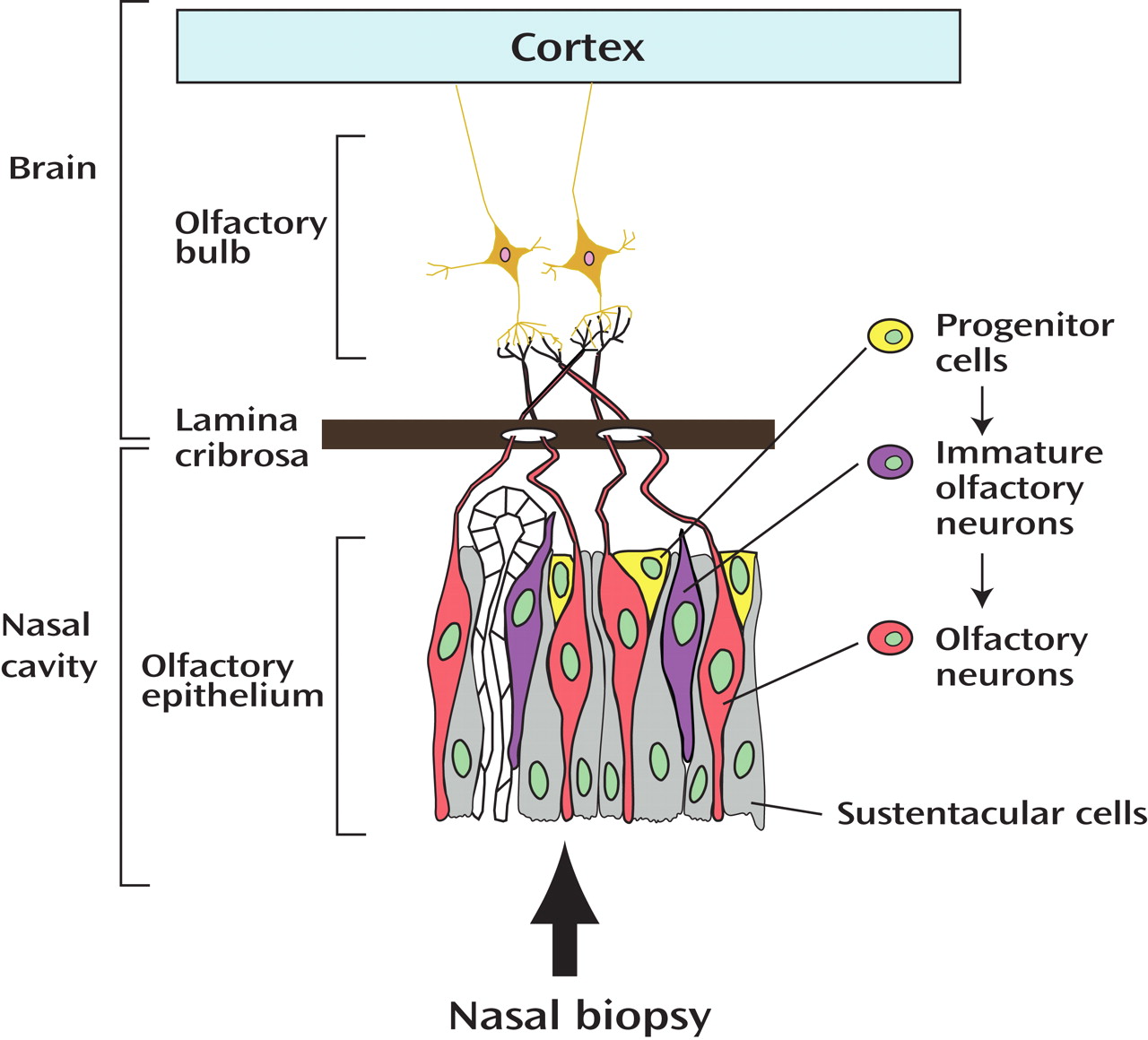
In order to make this suggestion a more solid foundation for future clinical and basic science investigation, further study of olfactory epithelium by means of nasal biopsy may be an important step. Olfactory epithelium is a unique tissue where neurons of central nervous system exist and undergo regeneration and differentiation, key features of neurodevelopment, throughout life
(1) . Thus, olfactory epithelium and its synaptic targets in the olfactory bulb provide an opportunity for a snapshot of morphologic and molecular neurodevelopmental processes even in adults. Recently, several groups have been successful in preparing neuronal cultures from biopsied olfactory epithelium
(8 –
10) . If the findings in this report
(7) are further validated by molecular studies using cultured olfactory neurons exposed to the same stimuli used in the clinical setting, their claim will be more solid.
Susceptibility of a specific signaling cascade associated with a key disease mechanism has been previously reported in other brain disorders. Huntington’s disease is caused by an expansion of glutamine repeat in the causal genetic factor Huntington, and mitochondrial abnormalities are reported in brains from patients with the disease. Increased susceptibility to mitochondrial toxin in the lymphoblasts from Huntington’s disease patients is known in a genetic mutation (expansion of polyglutamine)-dependent manner
(11) . By analogy, olfactory neuronal cultures from patients that show clinical deficits in detection threshold sensitivity to lyral, but not citralva, may display abnormal response of cAMP signaling to lyral. We may further explore a possible link of these phenotypes to genetic susceptibility factors for schizophrenia associated with cAMP signaling, such as disrupted-in-schizophrenia and the galphas subunit of heterotrimeric G-proteins
(12 –
14) .
The full clinical and genetic implications of these findings also have yet to be explored. Odor identification dysfunction is particularly associated with prominent and enduring negative symptoms of schizophrenia
(15) . Likewise, olfactory detection threshold sensitivity to some specific stimuli may be abnormal in a subtype of the disease. Intriguingly, a polymorphism of GNAS1, the gene coding for the galphas subunit of heterotrimeric G-proteins is associated with deficit schizophrenia
(14) . It would be important to explore olfactory detection threshold sensitivity as well as response of cultured olfactory neurons in association with molecular pathways involving genetic susceptibility factors to schizophrenia, by using more varieties of stimuli than citralva and lyral. Therefore, we may potentially develop various biomarkers that can be tested during a simple clinical examination and reflect etiology-based mechanisms of disease susceptibility and pathology.