Anorexia nervosa is characterized by severe emaciation, amenorrhea, and hyperactivity in ≥80% of patients with the disorder (
1). The mortality rate is the highest for any mental illness among young women (
2), and efficacy results of drug trials (
3) and psychotherapeutic interventions remain inadequate (
4). The etiology of anorexia nervosa is unknown. However, factors sustaining core symptoms of the disorder are important in understanding its chronicity. In this context, the interaction of stress and eating behavior has been the focus of theories based on research on self-starvation in animals (
5,
6). In response to stress, such as an increased level of physical activity (
7) or state of anxiety (
8), both animals and humans initially reduce caloric intake. If food is available, feeding behavior will adapt over the long run to an increased metabolic rate that compensates for any increased energy consumption. However, in rodents, if food remains restricted and the option of nonforced physical activity is given, self-starvation through excessive wheel running until death has been observed (
6).
Empirical evidence specific to self-starvation, showing that it does not refer to a deliberate reduction of food intake and is not dependent on reinforcement schedules, has been present in the literature since the 1950s (
6). Processes similar to those hypothesized in animal models are assumed in patients with anorexia nervosa. The auto-addiction opioid model (
9,
10) proposes that endogenous opioids functionally support adaption to starvation by conserving absorbed resources and by the concomitant decrease of the metabolic rate. Disturbance of these processes could result in a syndrome of starvation dependence in anorexia nervosa patients (
11), since opioids play a major role in addiction and reward (
12). This model is supported by the observation of increased endogenous opioid activity in the CSF of anorexia nervosa patients (
13), which is consistent with another theoretical approach (
14) stressing the relevance of the mesolimbic reward system of the brain (with the ventral striatum as a core structure), since its activity level is modulated by expectation or receipt of reward (
15,
16). Stress-related activation of the hypothalamic-pituitary-adrenal axis is responsible for activation of mesolimbic dopaminergic neurons in the ventral tegmental area projecting into the ventral striatum. Electrophysiological recordings in primates demonstrated that these neurons are easily conditioned to stimuli predicting reward (
17), and conditioned dopamine release in the ventral striatum has been shown in humans (
18). Further, ventral striatal dopamine secretion is provoked by stress-related secretion of cortisol (
19). In reaction to starvation-associated cues, these processes may contribute to the development of starvation dependence (
14). Although this theory, which stresses ventral striatal dopamine secretion, did not explicitly address opioid function, it has been shown that an increase of the opioid level in the ventral tegmental area is accompanied by increased dopamine release in the ventral striatum (
20).
Most of these findings are based on animal research, and empirical support for analogue processes in humans is sparse. However, there is growing evidence suggesting that reward-related processes are present in anorexia nervosa patients, with the mesolimbic reward system being essential in mediating maintenance of the disorder. Reduced concentrations of dopamine metabolites in the CSF of anorexia nervosa patients suggest an altered dopamine function (
21). In the ventral striatum, dopamine 2 and 3 (D
2/D
3) receptor binding is increased in recovered anorexia nervosa patients (
22). One recent study using monetary rewards (
23) focused on the role of the ventral striatum in recovered patients with anorexia nervosa. Contrary to comparison subjects, recovered anorexia nervosa patients did not show a pattern of differential activation in response to losing and winning money. Disturbances of the reward system in the acute state also have been investigated via the startle reflex paradigm, showing diminished appetitive processing of standardized positive stimuli in patients with anorexia nervosa (
24). Thus, altered dopaminergic function could affect the evaluation of reward stimuli in these patients, resulting in reduced responsiveness toward disease-nonspecific reward stimuli and increased reaction toward disease-specific stimuli (
25), such as cues for emaciation or cachexia.
In the present study, we examined ventral striatal activity in patients with anorexia nervosa in response to stimuli depicting features of underweight, normal weight, and overweight variants of a canonical female body. We predicted that an altered responsiveness of the ventral striatal reward system in anorexia nervosa patients, relative to healthy comparison subjects, would be evidenced by a differential activity pattern, rather than by pure hyporesponsiveness, upon processing these stimuli.
Method
Fourteen women diagnosed with anorexia nervosa according to DSM-IV (
26) were recruited from outpatient (N=5) and inpatient (N=7) services of the Department of Psychosomatic Medicine and Psychotherapy, University of Ulm, and the Clinic for Psychosomatic Medicine, Bad Grönenbach, Germany (N=2). Nine patients met criteria for restrictive subtype anorexia nervosa (subtype 1), and five patients engaged in binge eating/purging behavior (subtype 2). None of the patients had a lifetime diagnosis of bulimia nervosa. Exclusion criteria were a lifetime diagnosis of substance abuse or dependence, a lifetime diagnosis of psychotic disorder, and a diagnosis of major depression or anxiety disorder within 3 months of the start of the study. Five patients were receiving selective serotonin reuptake inhibitors (doxepine, 10 mg/day; fluoxetine, 20 mg/day; citalopram, 20 mg b.i.d.; and two patients taking escitalopram, 10 mg/day [potential influences of these five patients on the main results are discussed in the data supplement accompanying the online version of this article]). Fourteen healthy comparison women, who were group-matched for age, education level, and handedness, were recruited via local advertisements. Healthy comparison women had no lifetime or concurrent diagnosis of any psychiatric disorder, neurological disorder, or other serious medical illness and were medication-free. In order to diagnose major axis I disorders, all participants were assessed using the German equivalent (
27) of the Structured Clinical Interview for DSM-IV (SCID) (
28). Body weight and shape concerns among participants were evaluated using the Eating Disorder Examination (
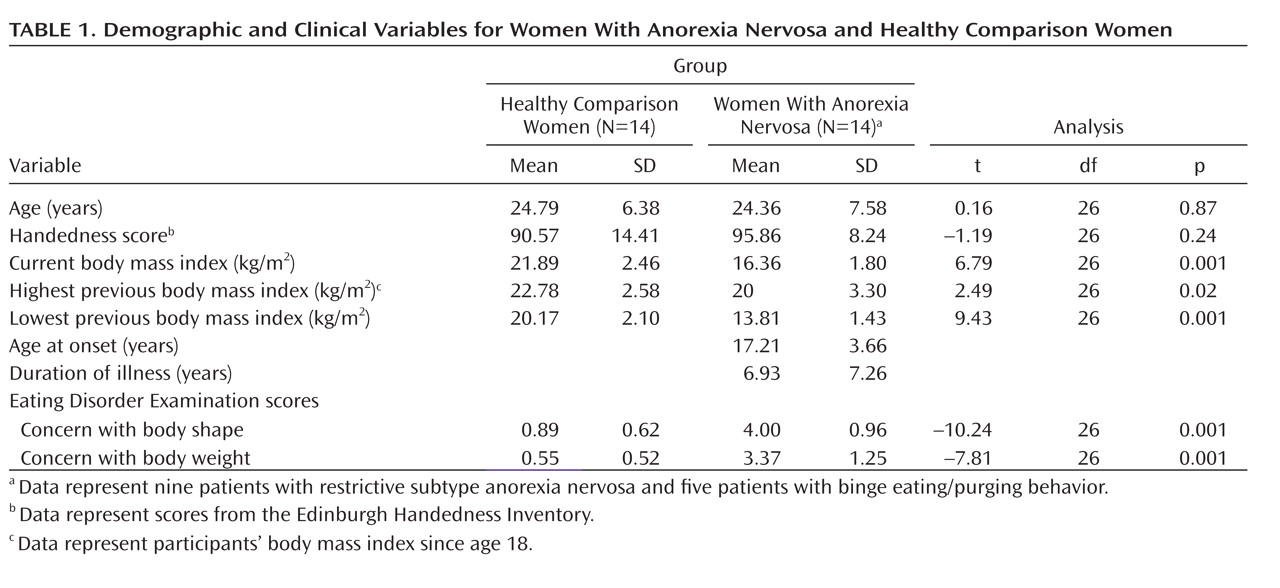
29). After complete description of the study was given, written informed consent was obtained. All methods and procedures were approved by the Human Subjects Committee of the University of Ulm and conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki). Anorexia nervosa patients had a significantly lower actual mean body mass index as well as significantly lower indices for lowest and highest previous body mass index relative to healthy comparison women (
Table 1).
Paradigm
The stimuli were 120 computer-generated nude female images of the same woman (height: 165 cm), varying in body mass index and body posture (
30), with 15°, 25°, 35°, and 45° deviations from the centered vertical body axis to the left or right. Images were assigned to one of the following three weight categories: underweight (body mass index: 12 to 16 kg/m
2), normal weight (body mass index: 19 to 23 kg/m
2), and overweight (body mass index: 26 to 30 kg/m
2), making up the three levels of the factor stimulus. Photorealistic textures were used to give a familiar presentation of specific features according to the particular body mass index. In the task referred to as "feel," subjects were required to process each stimulus in a self-referring way (i.e., "Imagine you have the same body shape as this woman. How would you feel? Watch the woman carefully, and transfer what you see to your own body.") and to give ratings of each stimulus using four buttons on a keyboard (scale: 1 ["very bad"] to 4 ["very good"]). In the control task referred to as "weight," subjects were required to estimate the weight of each body image shown according to one out of four weight categories (30 kg, 45 kg, 60 kg, 75 kg), selecting a category using a button-press keyboard. The entire scanning procedure consisted of four separate sessions, each containing 30 trials (15 "feel" and 15 "weight" tasks in randomized order). Each trial started with a fixation cross (average duration: 1,610 msec), followed by one of the two scales indicating the task ("feel" or "weight"; fixed duration: 2,500 msec each) for the ensuing stimulus (6,000 msec each), and the trial ended with a fixation cross (7,000 msec each). Stimuli were presented via liquid crystal display video goggles (Resonance Technologies, Northridge, Calif.).
Scanning Procedures
Blood-oxygen-level-dependent (BOLD) gradient echo-planar imaging was performed on a 3 Tesla Siemens Magnetom Allegra (Siemens, Erlangen, Germany). Twenty-five transversal slices were acquired, with a repetition time of 1,610 msec, an echo time of 35 msec, and a receiver bandwidth of 4,112 Hz/pixel in an ascending direction (matrix=64×64 pixels; pixel size=3×3 mm2; slice thickness=3 mm, with 0.75 mm gap). The partial image volumes covered all regions of the brain except the dorsal part of the parietal cortex. Axial slices were tilted at –25° relative to the anterior commissure-posterior commissure line in a transversal-to-coronary direction. Additionally, a full brain echo-planar image, with 35 slices and a sagittal magnetization-prepared rapid acquisition gradient-echo T1 structural image, was acquired (matrix=256×256 pixels, voxel size=1×1×1 mm3).
Functional Magnetic Resonance Imaging (fMRI)
Data analysis was performed using Statistical Parametric Mapping-5 (Release 748 [http://www.fil.ion.ucl.ac.uk/spm]). Each fMRI data series underwent slice-time correction and spatial realignment by aligning the first scan from each session with the first scan of the first session and aligning the images within sessions with the first image of a particular session. Time series were normalized to the standard Montreal Neurological Institute T1 template, with the coregistered individual T1 image as a reference. Volumes were resliced to a voxel size of 2×2×2 mm3 and spatially smoothed using a 10-mm full-width at half-maximum Gaussian kernel.
At the first level, design matrices of individual general linear models incorporated six regressors that combined the factor stimuli (underweight, normal weight, or overweight) with the task ("feel" or "weight"). Two additional regressors accounted for button presses and the appearance of scale ratings. Regressors were defined with onsets at the time of appearance of the corresponding event and convolved with the canonical hemodynamic response function. At the second level, group analysis was performed using analysis of variance (ANOVA), with one between-subjects factor and one within-subjects factor reflecting combinations of task-by-stimulus. A third factor, "subject," was added to the design matrix in order to remove variability as a result of differences in the participants' average responses.
Statistical analyses of relevant group-by-condition interactions were constrained to the bilateral ventral striatum, the region of interest, using the Wake Forest University PickAtlas software (Version 2.3, Wake Forest University School of Medicine, Winston-Salem, N.C. [see the data supplement accompanying the online version of this article]) (
31,
32). Only group-by-stimulus interactions were considered relevant for statistical analyses, since group comparisons of stimuli alone may have been confounded by nonspecific differences of activation magnitude in the region of interest in both groups (anorexia nervosa group and healthy comparison group). Using the normal-weight stimulus as a reference, the following two group-by-stimulus interactions were assessed: 1) the effect of the underweight body stimulus versus the normal-weight body stimulus on the ventral striatum and 2) the effect of the overweight body stimulus versus the normal-weight body stimulus on the ventral striatum. Both group-by-stimulus interactions were specified for both tasks ("feel" and "weight"). Inspection of the height of effect sizes for each of the resulting four tests was used to evaluate which of the group-by-stimulus interactions contributed to the overall significance (see the data supplement accompanying the online version of this article). As a nominal level of significance, a voxelwise threshold of p<0.05, with family-wise error correction for multiple comparisons, was used.
Results
Behavioral Data
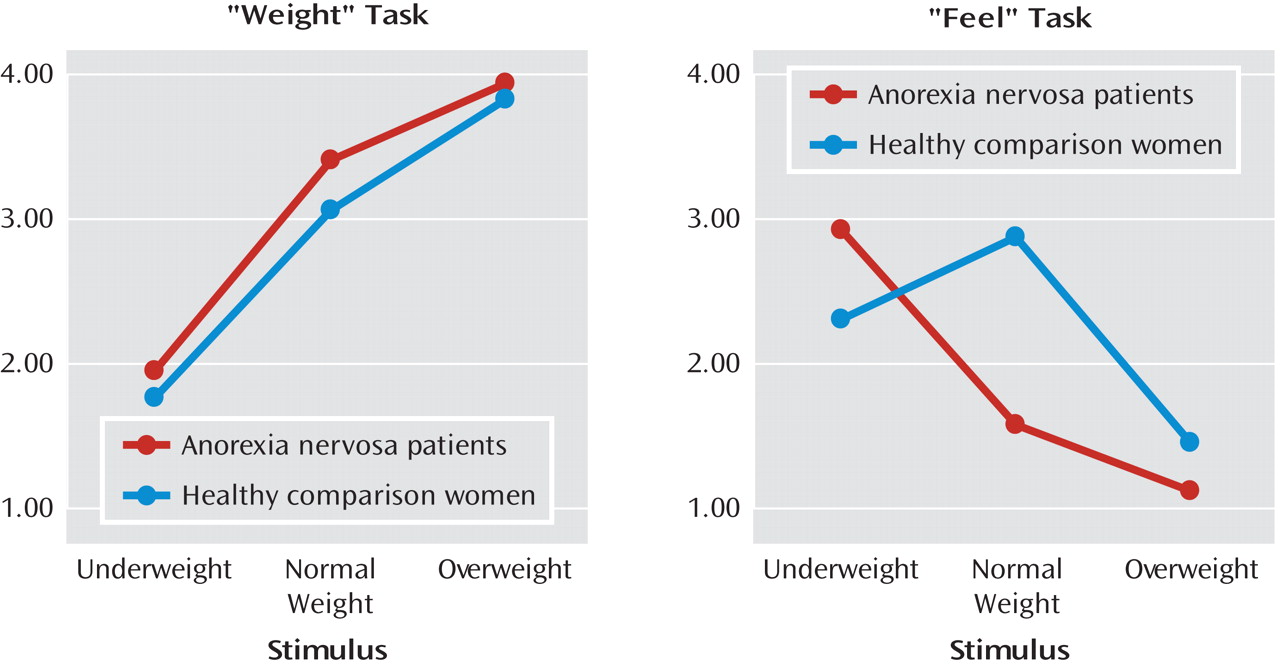
We utilized repeated-measures ANOVA for rating scores for the "weight" and "feel" tasks, which revealed a significant interaction of all main factors for group, task, and stimulus (F=33.98, df=2, 52, p=0.0001) (
Figure 1). Significant differences were mainly the result of ratings between the two groups for the "feel" task (F=36.63, df=2, 52, p=0.0001), not for the "weight" task. Bonferroni-corrected post hoc tests (nominal level of alpha: p<0.05) revealed no significant between-group differences for "weight" task ratings. However, for the "feel" task, analyses revealed that anorexia nervosa patients had higher positive scores relative to healthy comparison women for the underweight stimulus, reaching significance (p=0.02). Additionally, women with anorexia nervosa exhibited higher preference for the underweight stimulus when compared with their ratings of normal and overweight stimuli (all p values=0.0001). In contrast, women in the healthy comparison group showed a higher preference for the normal-weight stimulus (for the "feel" task) compared with that of anorexia nervosa patients (p=0.0001) and compared with their group's ratings of underweight (p=0.01) and overweight stimuli (p=0.0001).
There was no main effect of group on overall reaction times (F=0.41, df=1, 26, p=0.53; anorexia nervosa patients: 7.06 seconds [SD=0.24]; healthy comparison women: 7.12 seconds [SD=0.40]). Healthy comparison women showed relatively stable reaction times over all three factor stimulus categories (underweight, normal weight, and overweight). Women in the anorexia nervosa group reacted faster to overweight stimuli (p=0.0001) compared with underweight (p=0.0001) and normal-weight stimuli (p=0.004; effect of group-by-stimulus interaction: F=6.37, df=2, 52, p=0.003). This effect was not further modulated by the factor task.
fMRI Data
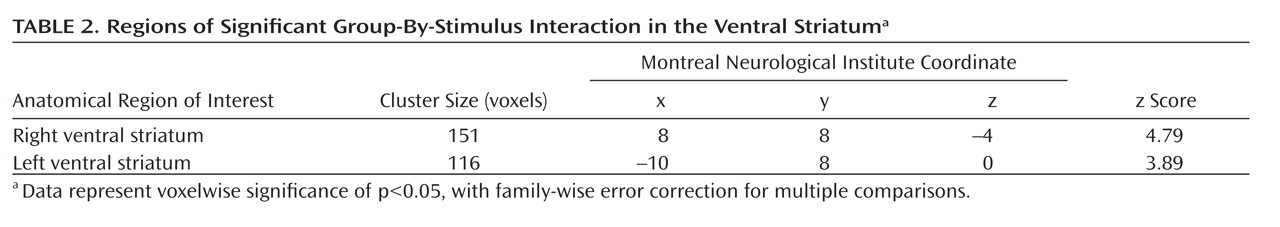
An F test for the two relevant group-by-stimulus interactions for the "feel" and "weight" tasks (the effect of the underweight body image versus the normal-weight body image and the effect of the overweight body image versus the normal-weight body image) yielded a significant effect in the bilateral ventral striatum (right ventral striatum: maximum peak at Montreal Neurological Institute coordinates: x=10, y=8, z=–2; z score=4.07, p<0.05, family-wise error corrected for multiple comparisons; cluster size=69 voxels; left ventral striatum: Montreal Neurological Institute coordinates: x=–12, y=10, z=2; z score=3.61, p<0.05, family-wise error corrected for multiple comparisons; cluster size=16 voxels). Group-by-stimulus interaction contrasting underweight stimuli against normal-weight stimuli during the "feel" task contributed most to the overall significance. For the "weight" task, this interaction also showed a significant effect, although of less height. Contrasting overweight stimuli against normal-weight stimuli did not contribute to the significant "F" value in the "feel" or "weight" task (see the data supplement accompanying the online version of this article for bar plots of each modeled effect). To further evaluate the effect of task on the significant group-by-stimulus interactions, an appropriate F test, which set the relevant contrast for the "weight" task relative to the "feel" task, did not produce any significant differences at the predefined level of significance of p<0.05, family-wise error corrected for multiple comparisons. The same F test produced significant voxels in the ventral striatum (right ventral striatum: Montreal Neurological Institute coordinates: x=8, y=12, z=–6; z score=2.01, p=0.02; cluster size=27 voxels; left ventral striatum: Montreal Neurological Institute coordinates: x=–8, y=12, z=–4; z score=1.99, p=0.02; cluster size=15 voxels), although with a more lenient threshold (p<0.05, uncorrected), with the effect of the group-by-stimulus interaction set higher for the "feel" task than for the "weight" task. Thus, further analysis of the group-by-stimulus interaction was constrained to the "feel" task only, in addition to the effects of the different task types not being part of the main focus of the study.
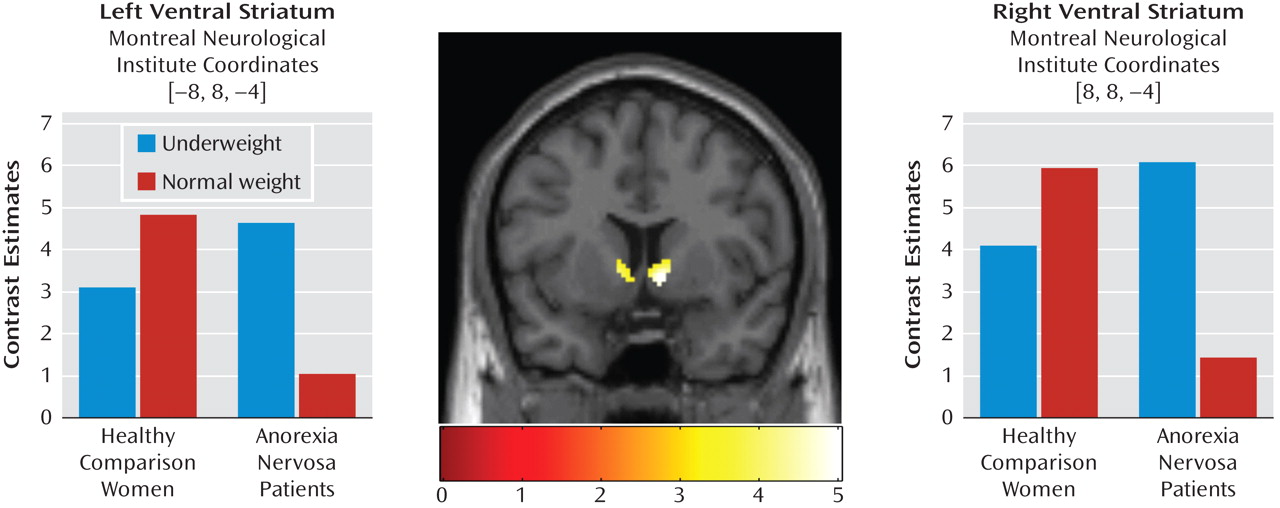
Subsequent single-tailed t-test contrasts examining the direction of significant group-by-stimulus interaction revealed higher ventral striatal activity in women with anorexia nervosa when processing underweight stimuli compared with normal-weight stimuli and in healthy comparison women when processing normal-weight stimuli compared with underweight stimuli (
Table 2,
Figure 2). Whole brain analysis at the same level of significance showed only the right ventral striatum, at Montreal Neurological Institute coordinates 8, 8, –4 (x, y, z, respectively), to be significant for this interaction. The inverted t-test contrast within the ventral striatum did not yield any significant effects (p>0.05, uncorrected). Within-group differences of neural activations in response to underweight and normal-weight stimuli were tested for significance, with the significant ventral striatal voxels of the group-by-stimulus interaction as a functional mask. In women with anorexia nervosa, all voxels revealed higher activity in response to underweight stimuli compared with normal-weight stimuli, with the smallest difference significant at p=0.004 in the right ventral striatum and p=0.03 in the left ventral striatum. In healthy comparison women, higher activity in response to normal-weight stimuli compared with underweight stimuli was significant only in a subset of voxels (right ventral striatum: 40.4%, with reference to the cluster extent of the group-by-stimulus interaction [Table 2]; minimum p value=0.05; left ventral striatum: 63.8%; minimum p value=0.05).
Discussion
Based on animal models of anorexia nervosa, we predicted altered activity patterns in the ventral striatal reward system in women with anorexia nervosa, depending on altered processing of disease-specific visual stimuli depicting a female body image with underweight, normal weight, and overweight canonical whole-body features according to standardized body mass indices.
Behaviorally, women with anorexia nervosa exhibited significantly increased positive ratings in response to underweight stimuli than in response to normal-weight stimuli, while group-matched healthy comparison women exhibited the reverse pattern when the task was performed in a self-referent way. On a control task requesting weight estimations of each stimulus, women with anorexia nervosa and healthy comparison women did not differ with regard to correctness or decision times. Functionally, group-by-stimulus interactions were highly significant in the bilateral ventral striatum. Underweight stimuli elicited the highest ventral striatal activity in women with anorexia nervosa, while normal-weight stimuli produced the highest activity in healthy comparison women. Differential responses to overweight stimuli did not substantially contribute to the overall effect, either behaviorally or functionally, on either task ("feel" or "weight"). The magnitude of ventral striatal activity, in response to normal-weight stimuli among the comparison group and underweight stimuli among the anorexia nervosa group, was comparable between both groups, which does not support general hyporesponsiveness of the ventral striatum in anorexia nervosa patients. Further, within-group differences of ventral striatal activity in response to underweight and normal-weight stimuli were markedly lower in healthy comparison women than in women with anorexia nervosa, which might reflect a qualitative feature differentiating healthy comparison women from women with acute anorexia nervosa.
This pattern of activation is in agreement with previous assumptions (
22,
25) concerning differential responsiveness to disease-specific and disease-nonspecific stimulation among anorexia nervosa patients. One previous study examining the processing of monetary wins and losses (
23) reported an absence of a differential ventral striatal response in anorexia nervosa patients. However, anorexia nervosa patients showed an increased activation in the prefrontal and parietal cortices, which was interpreted as cognitive strategic control in order to cope with the uncertainty inherent in the task. In the present study, the differential ventral striatal interaction was not accompanied by differential activation of "cognitive" cortices, even at decreased significance levels. Although interpretation of an absent effect should be treated with caution, these different results might relate to differences in both the uncertainty of the task and the reward values of the stimuli.
The absence of differential ventral striatal activation toward disease-nonrelated rewards but the presence of differential activation toward disease-related stimuli is consistent with theories of starvation dependence in anorexia nervosa patients (
9,
11,
14,
33). These theories stress the role of reward-related brain structures modulated by endogenous opioids and dopamine, since there are abnormalities concerning both endogenous opioids and dopamine in anorexia nervosa patients. Within the ventral striatum, dopamine is shown to influence appetence, while opioids modulate hedonic aspects of the reward response (
34). Accordingly, clinical observations and case reports point to positive experiences of starvation among individuals whose circumscribed pleasure and obsession in life almost exclusively relates to the maintenance of cachexia (
35,
36). Positive experiences of the self-emaciated body are also a main topic of "pro-ana" communities on the Internet, which promote anorexia nervosa as a desirable lifestyle choice (
37).
Focus on operant rewards may be further supported by avoidance of potentially harmful stimulation, since the mere absence of negative affect may be a strong motivator for the development and maintenance of symptoms (
25,
38). Increased levels of harm avoidance in anorexia nervosa patients are assumed to contribute to dietary behavior, since this alters activity of the serotonergic system, resulting in reduced negative affectivity (
25).
Although smaller in its height of effect, the group-by-stimulus interaction contrasting underweight and normal-weight stimuli in the present study was evident when subjects were instructed to rate the weight of each stimulus. Additionally, a three-way interaction of factors (group, stimulus, and task) only indicated a greater effect for the "feel" task than the "weight" task, suggesting that the stimuli per se were controlling the interaction of interest, and thus it appears that a self-referent task instruction did not represent a necessary condition to obtain the present results. This might indicate that the stimuli induced a positive experience of starvation independent of further processing related to task instructions. However, previous studies (
39,
40) did not report ventral striatal activity upon processing of body stimuli among individuals with eating disorders, which would have supported the hypothesis that starvation dependence in humans is induced by pure presentation of body stimuli, although there might be different reasons for the absence of effect (e.g., type of stimuli used, focus on aversive rather than appetitive processing, and different theoretical framing).
There are several limitations to the present paradigm. First, a spillover may have occurred between randomized tasks such that a clear explanation regarding the effect of the task instruction on the triggering of starvation experience cannot be provided. Second, sample sizes for restrictive subtype anorexia nervosa and binge eating/purging behavior were not sufficient to examine differences between the two subtype groups. Compared with the binge eating/purging subtype, more pronounced aberrations in dopamine function have been indicated in anorexia nervosa patients recovered from the restrictive subtype (
21). Future studies using larger subtype sample sizes may be able to determine whether different subtypes contribute to a different neural signature in terms of differences in ventral striatal activity. Additionally, the present results do not provide an explanation pertaining to the functional role of dopaminergic neurotransmission in disease development and its modulating role for therapeutic progression. In this context, longitudinal studies in combination with repetitive neuroimaging of the ventral striatum would be beneficial.
In summary, the present results show that the ventral striatal reward system is differentially activated upon processing of disease-specific stimulation in patients with acute anorexia nervosa. These findings are in accordance with predictions derived from animal studies on the pivotal role of the human reward system. Thus, our results support theories of starvation dependence accounting for the maintenance of anorexia nervosa, which may lead to an expansion of current concepts of intervention for the disorder.