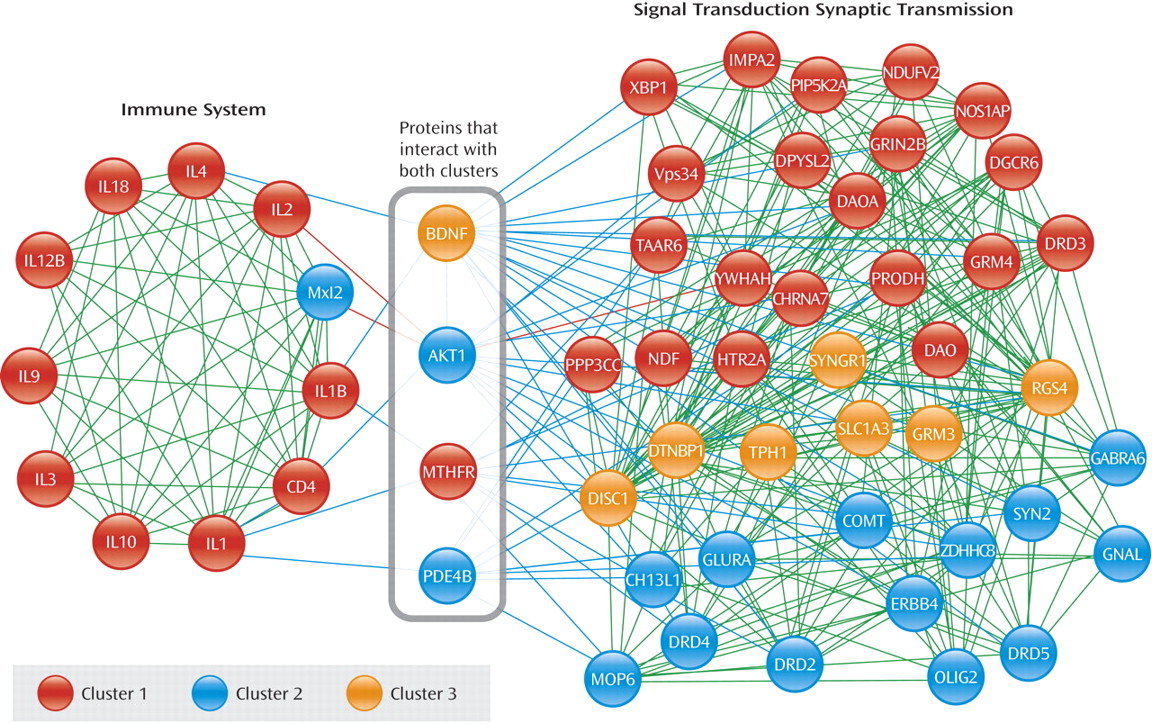
The use of publically available, regularly updated,
bioinformatic tools to explore disease-related findings in psychiatric illnesses is an emerging methodology. In the present study, a series of bioinformatic tools was used to perform an unbiased analysis of gene alterations associated with schizophrenia. All analyses were performed using data already in the published literature. As the first step, two Internet databases (MEDLINE and SZGene) were used to search for and identify all genes potentially associated with schizophrenia. The initial search resulted in 744 single nucleotide polymorphisms (SNP) or copy number variations (CNV), of which 535 SNPs/CNVs met the criterion of a p≤0.05 association with any phenotype of schizophrenia in a published study. Next, using STRING, the protein products of these 535 genes were identified. In addition, since STRING identifies not only the protein products of genes but also their physical interactions with other proteins in biological pathways, it generates a “functional association” database. Of all 535 gene products, 417 proteins were shown to have probable relationships between each other, with a total of 2,183 interactions between the 417 protein products. The next bioinformatics program, MCODE, generated information about the closeness of biological function across the 417 related proteins and found six protein clusters of functionally related proteins. The three most strongly related clusters were significantly associated at a p<0.005 level, denoting proteins that are functionally related to each other with high certainty. Finally, the three most highly related protein clusters were subjected to an analysis with a microarray annotation tool (MAP), which annotated the interconnections between the proteins identifying clusters of proteins related to a biological function. MAP integrated these three most significant clusters with each other, generating two functionally integrated but largely independent clusters: one of immune-related proteins and another of neuronal signaling proteins. MAP also showed that there are four protein products that have interactions with the two functionally-related clusters—brain-derived neurotrophic factor (BDNF), V-akt murine thymoma viral oncogene homolog 1 (AKT1), methylenetetrahydrofolate reductase (MTHFR), and phosphodiesterase 4B (PDE4B)—proteins that themselves have been associated with schizophrenia. The implications of these kinds of data for schizophrenia suggest the possibility that immune system proteins as well as signal transduction and transmission proteins could be relevant for the understanding of schizophrenia, possibly implicating some specific function for the four “dual interacting” proteins. (The gene and protein names in the figure are included in the data supplement accompanying the online version of this article.)
Definitions of abbreviations are available in the data supplement accompanying the online version of this article (http://ajp.psychiatryonline.org/cgi/content/full/166/8/854/DC1).