Aside from its clinical importance in genetic counseling, the characterization of sibling recurrence is pivotal in the elucidation of mechanisms of inheritance for any genetically influenced condition. Categorical estimates of recurrence risk have previously indicated that the siblings of probands with autistic disorder have a 22-fold relative risk of developing the disorder (
1). However, recent discoveries in the field of autism research have suggested that there exists a diversity of genetic mechanisms that give rise to the autistic syndrome (
2), that each mechanism is associated with its own pattern of intergenerational transmission, and that autistic symptoms exhibit a wide, continuous distribution both in the general population and among clinically ascertained cases (
3–5). An additional complexity of the quantitative variation of autistic symptoms is that when considering samples largely comprising sporadic (nonfamilial) cases of autism, there appear to exist—within the families—separable, discrete populations of affected and unaffected children whose respective severity distributions partially overlap (
6,
7). Thus, a reexamination of the phenomenon of recurrence accounting for these developments is warranted.
For the 10%–20% of all autism cases whose origins are attributable to known genetic causes, there is an emerging understanding of how specific molecular mechanisms of transmission might map to a given pattern of recurrence in families. For example, large,
de novo chromosomal rearrangements (mutations of typically major effect) have been observed in some 10% of children with autism (
8,
9), compared with substantially lower rates in the general population. Common allelic variations of small but statistically significant effect have been associated with incremental increases in susceptibility to autism, primarily among multiple-incidence autism families (
10–12). Rare mutations in a number of synapse-related genes, singly or in combination (
13), have also been associated with a diverse array of full and intermediate autism phenotypes.
It is with this background that the present clinico-epidemiologic family study attempts to advance understanding of the relative proportions of autism cases in the population that might be attributable to these various mechanisms of genetic transmission, recognizing that the vast majority of cases of autism currently remain idiopathic. This study has the following two primary objectives: 1) to derive an updated estimate of recurrence risk in a large, volunteer registry of autism-affected families in which the children were both categorically and quantitatively characterized and 2) to explore the distributions of quantitative (subclinical) autistic traits in families with and without categorically defined recurrence. Additionally, the study presents the opportunity to consider (in a large sample) whether any aggregation of language delays in autism-unaffected children might constitute an additional type of recurrence among siblings in some families. Lindgren et al. (
14) recently summarized the existing literature on the aggregation of language impairment in first-degree relatives of children with autism spectrum disorder and reported additional data on 52 families. Their findings generally supported estimates of 20%–25% from prior studies of comparable sample size (
15,
16), with a greater predominance of pragmatic versus structural language deficits.
Results
Recurrence rates—conservatively operationalized as the occurrence of an autistic syndrome in one or more siblings of an index case, using as a denominator all additional children in the family (whether earlier- or later-born)—are presented in
Table 2 as a function of recurrence definition. We note that use of standardized quantitative definitions of recurrence resulted in a pronounced shift in the gender ratio for recurrence events. Categorical autism spectrum disorder status in an additional child occurred in 10.9% of the families (8.2% of the individual children in the entire sibling pool). An additional 20% of presumed-unaffected siblings had a history of language delay, and of those, 54% exhibited autistic qualities of speech ascertained by the Social Communication Questionnaire, resulting in a total of 8.9% exhibiting a history of language delay with autistic speech.
The family-based recurrence rate was uniformly higher than that calculated for all individual siblings in the sample, which reflects the possible effects of stoppage (a tendency for families who have a child with a serious clinical condition to reduce subsequent childbearing). When considering families with more than two children affected by an autism spectrum disorder and the existence of at least one additional sibling, autism status in a third child occurred in 8% of these families (for the entire Interactive Autism Network registry, this figure was 18%). Linear regression revealed statistically significant effects of both proband and sibling gender on sibling standardized quantitative trait scores on the Social Responsiveness Scale (F=5.80, df=4, 622, p<0.001; R2=0.036). However, the effects were very modest in magnitude, and there was no appreciable effect of proband level of functioning (non-verbal status or IQ <56) on sibling scores.
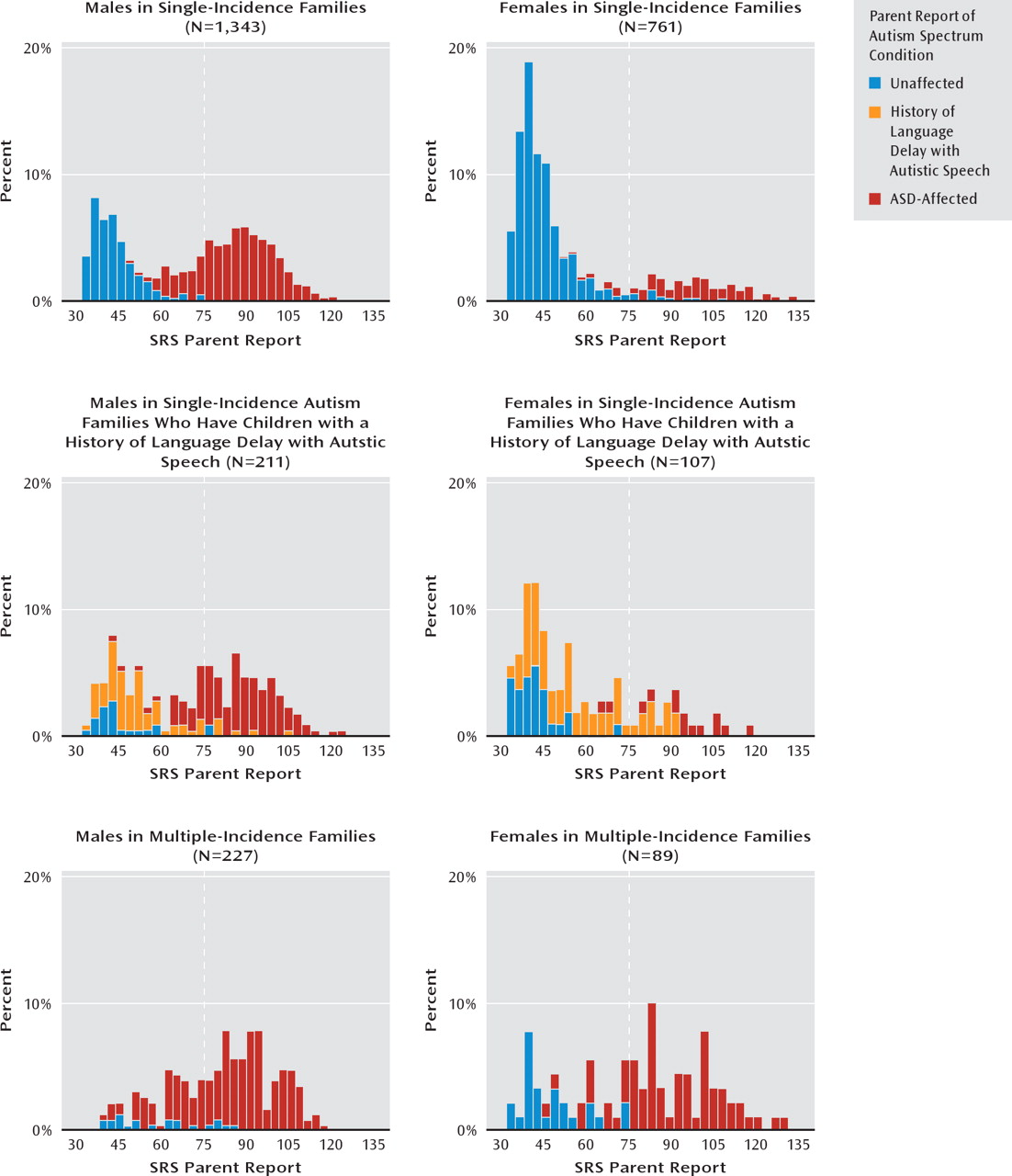
Table 3 lists the means and standard deviations for the quantitative trait scores of specific groupings of index cases and siblings, segregated by gender and family type. The respective quantitative trait distributions are depicted in the histograms presented in
Figure 1. Most striking across all subject groups and in keeping with our previous report (
7), we observed an absence of quantitative autistic traits in the unaffected siblings of autism-affected children in single-incidence families. Also in keeping with our previous report, we observed a relative aggregation of quantitative autistic traits in the unaffected siblings in multiple-incidence families. This was manifested by an elevated mean and a contrasting shape of the distribution, especially for presumed-unaffected male children in those families. Unaffected siblings with a history of language delay with autistic speech contributed to an intermediate distribution, with quantitative trait scores of unaffected female children significantly overlapping with those of autism-affected girls. The differences in mean Social Responsiveness Scale scores of presumed-unaffected siblings across the three groups were highly statistically significant and remained so for boys when multiple-incidence families were directly compared with single-incidence families.
The quantitative distributions depicted in the histograms in
Figure 1 reveal the manner in which parents distinguished their children with versus without an autism spectrum disorder diagnosis in this sample. The nadir in these distributions, effectively the point at which parents (on average) differentiated affected versus unaffected children in their families, fell below the Social Responsiveness Scale cutoff T score of 75. We also observed that the distributions for unaffected children in simplex families were slightly nonpathologically shifted relative to previously published general population distributions (
3) suggestive of subtle rater contrast effects. In the entire sample, there were 207 families with more than one autism-unaffected child for whom parent-reported Social Responsiveness Scale data were available. For these unaffected children (predominantly from single-incidence autism families in the sample), the sibling correlation for parent-reported data was 0.38, in keeping with previously published estimates (
3,
29).
Discussion
The results of this study of recurrence are notable in several respects and provide new information on the genetic epidemiology of autism spectrum conditions. First, there exists an aggregation of quantitative autistic traits among unaffected children in multiple-incidence autism spectrum disorder families—most pronounced in boys—but an absence of such traits in most single-incidence families, as initially observed in a prior study that included independent ratings by teachers, involving a smaller number of single-incidence families (
7). The absence of such aggregation in single-incidence families is also consistent with our recent taxometric analysis of the entire Interactive Autism Network data set (a predominantly single-incidence sample), identifying categorical discontinuity (autism spec-t rum disorder versus nonautism spectrum disorder) rather than graded levels of symptoms within this predominantly single-incidence family sample (
6). Second, we observed minimal effects of proband gender and level of functioning on the rate of sibling recurrence, but when using standardized quantitative criteria for designation of affected status, many more girls are identified, and the male:female ratio narrows to 3:2. Third, across all family types and highly consistent with prior family studies, some 20% of presumed-unaffected siblings carry a historic diagnosis of language delay, over one-half of whom exhibit distinctly autistic speech. This may constitute a form of recurrence in a substantial minority of autism-affected families. Finally, the rate of sibling recurrence of categorically defined autism spectrum disorder in this sample is in keeping with prior estimates, although distinctly lower than that reported for nonidentical twins from the same registry (31% concordance rate reported by Rosenberg et al. [30]). Whether this difference is explainable on the basis of 1) factors that might raise recurrence risk in twins versus 2) ascertainment bias favoring the enrollment of concordant over discordant twin pairs in this volunteer register will be a critical issue to resolve via future research in independent samples.
In summary, we observed a range of manifestations of sibling recurrence in autism to include 1) categorically defined autism spectrum disorder, 2) history of language delay with autistic speech qualities, and 3) aggregation of quantitative (subclinical) autistic traits. The third manifestation appears to be absent in most single-incidence autism families. These disparate manifestations of recurrence may reflect differential mechanisms of genetic transmission of autism in the population, which include (respectively) 1) rare recessive or de novo mutations (including chromosomal rearrangements) of substantial effect, which in some cases have accounted for sporadic incidence of autism; 2) inherited mutations that may be variably expressed and result in varying degrees of social and language impairment (i.e., categorically defined autism spectrum disorder, history of language delay with autistic speech) and/or sub-clinical autistic impairment; and 3) common susceptibility alleles or rare variants of minor effect, which may operate in additive or epistatic fashion. We note that even among single-incidence families in which affectation status appears categorical, the distribution of quantitative trait scores for affected children extends well into the range of the distribution for the general population. Thus, the continuum observed for autistic symptoms in nature may be composed of highly overlapping segments, each with its own mechanism (or mechanisms) of genetic transmission. Finally, the observation of a narrowing of the gender ratio when standardized quantitative criteria for affectation status are applied suggests the possibility that affected female children may be underascertained when using traditional categorical methods for diagnostic assignment.
Limitations of the study are that the sample was not fully epidemiologic (rather, a large volunteer register), not fully representative of the ethnicity of the population of U.S. children affected by autism, and the data were provided exclusively by parents, which potentially introduces a variety of biases, including rater contrast effects. Higher levels of rater contrast are expected in parent-reported data in clinically ascertained families (a reason for the use of teacher-reported data in our previous study [7]) and may have actually resulted in underestimation of the magnitude of familial aggregation among multiple-incidence families in this report. We also note that we were unable to directly compare the proportion of children with language delay in this sample with a population-based sample in which the same ascertainment methods were employed.
However, several aspects of the data validate the reports of parents, including the very high rate of reported diagnostic confirmation (98%) in families whose children underwent standardized testing for autism spectrum disorder (
18), the fact that parents' reports of a diagnosis corresponded closely to quantitative characterizations of social deficiency in their children (with nadirs closely corresponding to established cutoff scores for clinical-level symptoms), and that these results replicate what was observed by both teacher-reported data (minimizing the likelihood of rater contrast) and parent-reported data in a smaller independent sample (
7). It is important to note that elevations in quantitative autistic traits ascertained by the Social Responsiveness Scale and Social Communication Questionnaire have been observed in samples of children seriously affected by other primary psychiatric conditions not ascertained in the Interactive Autism Network data collection (
31,
32). Future research will need to explore the extent to which the quantitative distribution of autistic traits in these populations represent distinct or overlapping continua with those traits that characterize autism spectrum disorder.
On the basis of these findings, we propose careful reconsideration of what constitutes recurrence (and therefore diagnosis), informed by an understanding of the range of symptoms that aggregate in the siblings of autism-affected probands (including girls or twins) (
30), and that may more closely correspond to the manner in which autistic syndromes are intergenerationally transmitted. Among families of autism subjects in this sample, fully 21.7% exhibited a recurrence of either an autism spectrum disorder or a history of language delay with autistic speech, with a broad distribution of subclinical autistic traits among unaffected male subjects in multiple-incidence families.
Studies examining the association between autistic phenotypes and their underlying genetic (
33) or neurobiologic (
34,
35) determinants may be optimized by including information about recurrence of the autistic syndrome and the aggregation of relevant subclinical phenotypes among first-degree relatives. The data from the current study provide new perspectives on the relative proportions of autism cases in the general population that manifest distinct patterns of familial aggregation and should alert clinicians to the presence of both clinical and sub-clinical autism spectrum disorder-related-syndromes that occur in the siblings of children affected by autism.