Children of Depressed Mothers 1 Year After Remission of Maternal Depression: Findings From the STAR*D-Child Study
Abstract
Objective:
Method:
Results:
Conclusions:
Method
STAR*D and STAR*D-Child Studies
Sample
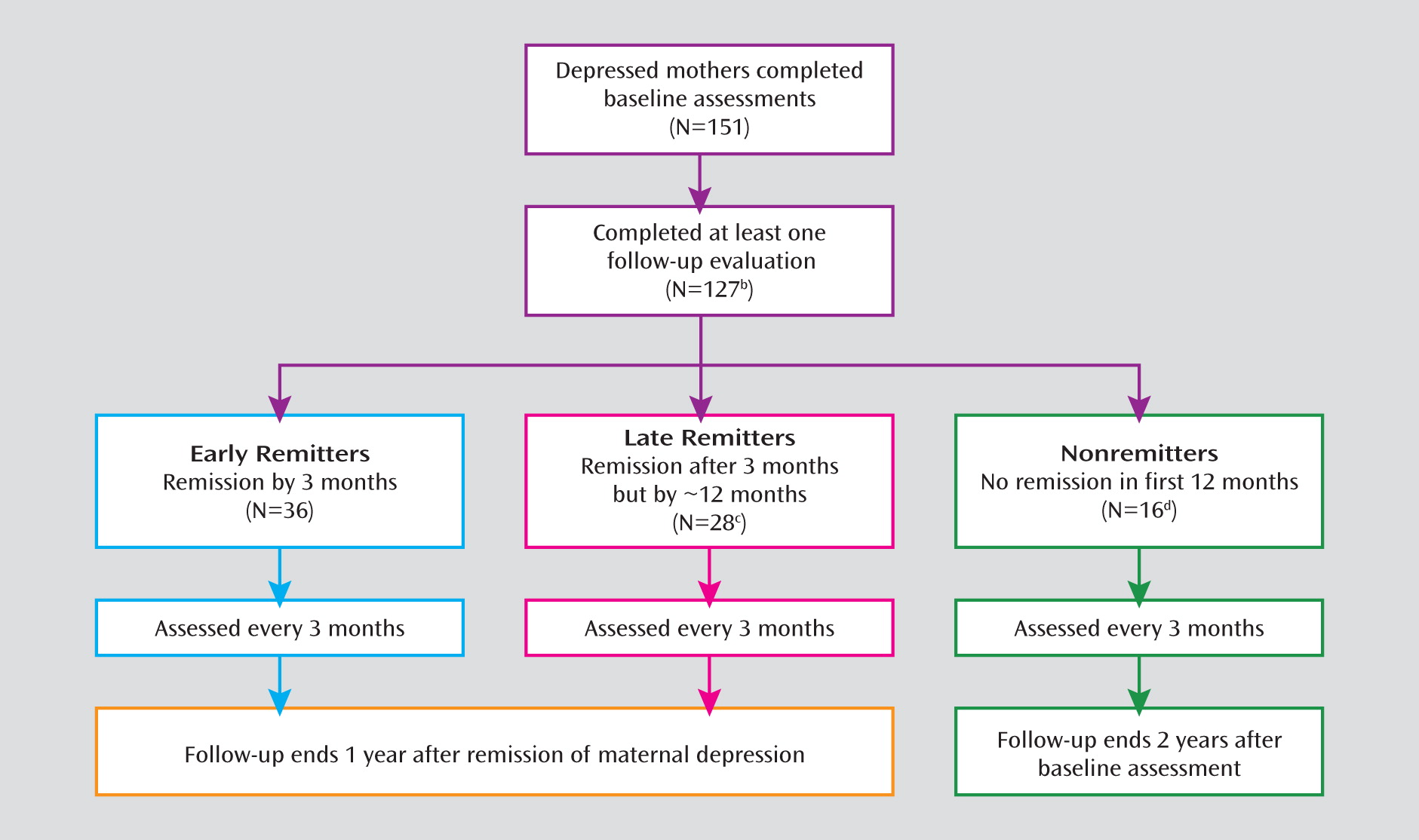
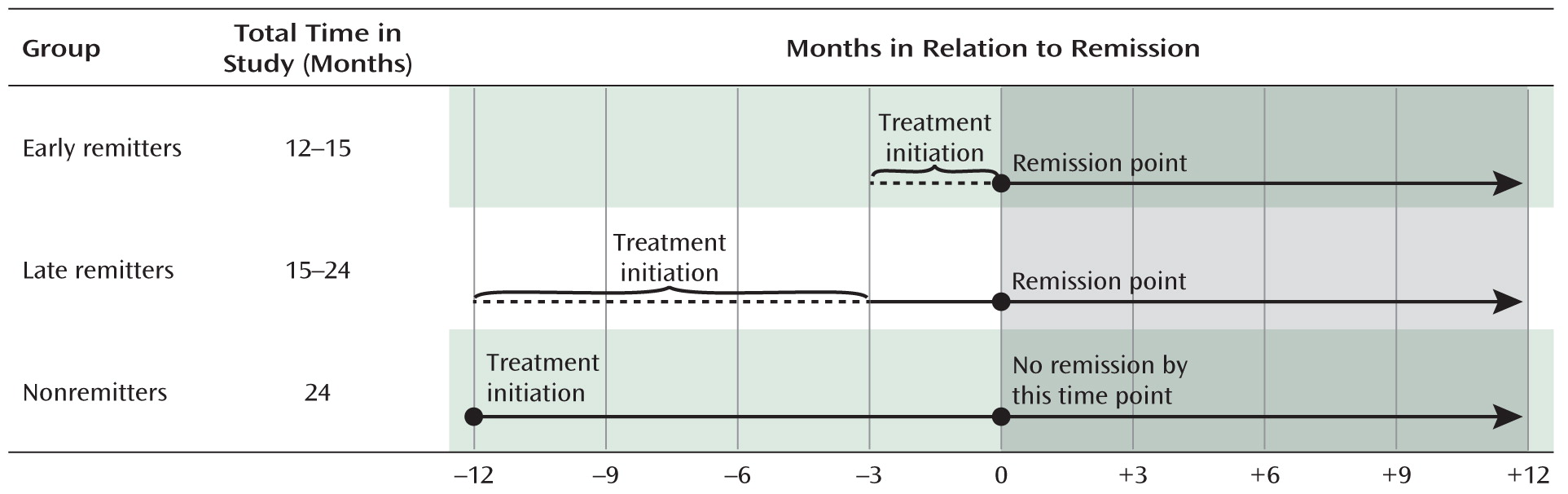
Assessments and Measures
Data Analysis
Results
Baseline Characteristics of Depressed Mothers and Their Children
| Subject Group and Variable | Early Remitters (N=36) | Late Remitters (N=28) | Nonremitters (N=16) | Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ANOVA F | df | p | |
| Mothers | |||||||||
| Age (years) | 39.0 | 6.8 | 36.9 | 6.3 | 40.3 | 6.6 | 1.52 | 2, 77 | 0.22 |
| Hamilton Depression Rating Scale score | 21.8 | 4.2 | 23.8 | 5.3 | 26.9 | 4.5 | 6.79 | 2, 77 | 0.002 |
| N | % | N | % | N | % | χ2 | df | p | |
| Race | 0.77a | ||||||||
| African American | 10 | 27.8 | 11 | 39.3 | 6 | 37.5 | 6 | ||
| White | 16 | 44.4 | 13 | 46.4 | 7 | 43.8 | |||
| Hispanic | 7 | 19.4 | 4 | 14.3 | 3 | 18.8 | |||
| Other | 3 | 8.3 | 0 | 0.0 | 0 | 0.0 | |||
| Education | 3.51 | 2 | 0.17 | ||||||
| Less than high school | 4 | 11.1 | 3 | 10.7 | 3 | 18.8 | |||
| High school (<college) | 19 | 52.8 | 20 | 71.4 | 11 | 68.8 | |||
| ≥College | 13 | 36.1 | 5 | 17.9 | 2 | 12.5 | |||
| Annual household income | 7.69 | 2 | 0.02 | ||||||
| Less than $15,000 | 4 | 11.4 | 7 | 25.9 | 5 | 33.3 | |||
| $15,000 to $39,999 | 11 | 31.4 | 11 | 40.7 | 7 | 46.7 | |||
| $40,000 or greater | 20 | 57.1 | 9 | 33.3 | 3 | 20.0 | |||
| Marital status | 0.047a | ||||||||
| Currently married | 23 | 63.9 | 10 | 35.7 | 4 | 25.0 | |||
| Separated or divorced | 4 | 11.1 | 5 | 17.9 | 5 | 31.3 | |||
| Never married | 9 | 25.0 | 13 | 46.4 | 7 | 43.8 | |||
| N | % | N | % | N | % | pa | |||
| Offspring | |||||||||
| Boy | 24 | 66.7 | 11 | 39.3 | 9 | 56.3 | 0.09 | ||
| Girl | 12 | 33.3 | 17 | 60.7 | 7 | 43.8 | |||
| Mean | SD | Mean | SD | Mean | SD | ANOVA F | df | p | |
| Age (years) | 12.0 | 2.8 | 11.4 | 2.5 | 12.6 | 2.7 | 1.17 | 2, 77 | 0.32 |
| K-SADS-PLb | |||||||||
| Symptoms (child report) | 5.4 | 5.9 | 6.0 | 4.3 | 7.7 | 5.8 | 3.28c | 2 | 0.19 |
| Symptoms (mother report) | 4.6 | 4.3 | 6.0 | 3.6 | 8.7 | 5.9 | 6.77c | 2 | 0.03 |
| Children's Global Assessment Scale | 70.6 | 11.1 | 68.4 | 15.3 | 66.1 | 10.2 | 0.72 | 2, 76 | 0.49 |
| Children's Behavior Checklist | |||||||||
| Total problems score | 57.7 | 8.4 | 55.1 | 11.7 | 53.6 | 10.0 | 1.11 | 2, 77 | 0.33 |
| Internalizing problems score | 58.1 | 8.4 | 55.9 | 10.2 | 53.3 | 11.2 | 1.39 | 2, 75 | 0.25 |
| Externalizing problems score | 55.4 | 8.5 | 54.6 | 11.4 | 52.3 | 9.3 | 0.55 | 2, 77 | 0.58 |
Child Outcomes and Maternal Remission Status
| Early Remitters | Late Remitters | Nonremitters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Score | Score | ||||||||||
| Measure and Assessment Point | N | Mean | SD | Time Trend (Beta) | N | Mean | SD | Time Trend (Beta) | N | Mean | SD | Time Trend (Beta) |
| Child-reported symptomsb | –0.039** | –0.035* | –0.009 | |||||||||
| 0 | 34 | 3.82 | 3.46 | 28 | 4.36 | 3.55 | 15 | 5.53 | 4.26 | |||
| 3 | 30 | 3.23 | 4.55 | 25 | 4.72 | 3.63 | 14 | 5.93 | 5.84 | |||
| 6 | 28 | 2.71 | 3.46 | 21 | 3.38 | 3.11 | 14 | 5.79 | 5.75 | |||
| 9 | 27 | 2.63 | 3.59 | 19 | 3.05 | 2.70 | 11 | 6.27 | 4.67 | |||
| 12 | 26 | 2.85 | 3.39 | 5 | 2.40 | 2.19 | 7 | 3.14 | 2.97 | |||
| Mother's report of children's symptomsb | –0.024* | –0.035* | –0.010 | |||||||||
| 0 | 34 | 3.68 | 3.17 | 28 | 5.14 | 4.12 | 15 | 7.13 | 6.17 | |||
| 3 | 30 | 3.37 | 3.87 | 25 | 4.32 | 3.89 | 14 | 7.21 | 6.60 | |||
| 6 | 28 | 2.89 | 3.02 | 21 | 3.71 | 3.64 | 14 | 6.50 | 6.71 | |||
| 9 | 27 | 3.04 | 3.33 | 19 | 3.21 | 3.38 | 11 | 7.64 | 6.30 | |||
| 12 | 26 | 3.00 | 3.38 | 5 | 4.60 | 4.98 | 7 | 5.43 | 5.50 | |||
| Child Behavior Checklist | ||||||||||||
| Total problem scorec | –0.315** | –0.271 | 0.187 | |||||||||
| 0 | 34 | 45.65 | 10.27 | 28 | 49.14 | 11.64 | 15 | 54.40 | 13.90 | |||
| 3 | 30 | 44.57 | 11.26 | 26 | 47.15 | 12.98 | 14 | 55.71 | 12.80 | |||
| 6 | 28 | 44.54 | 8.32 | 21 | 46.48 | 13.16 | 13 | 54.92 | 11.64 | |||
| 9 | 27 | 43.41 | 10.81 | 19 | 45.89 | 9.80 | 11 | 53.73 | 12.43 | |||
| 12 | 26 | 42.04 | 10.78 | 5 | 50.20 | 14.75 | 7 | 52.00 | 11.36 | |||
| Internalizing problem score | –0.254* | –0.305d | 0.120 | |||||||||
| 0 | 33 | 46.79 | 8.76 | 27 | 50.85 | 10.54 | 15 | 54.53 | 11.83 | |||
| 3 | 30 | 47.23 | 9.35 | 26 | 48.58 | 11.62 | 14 | 56.50 | 11.35 | |||
| 6 | 28 | 47.46 | 7.69 | 21 | 49.24 | 11.47 | 13 | 54.23 | 8.70 | |||
| 9 | 27 | 46.74 | 9.76 | 18 | 47.67 | 9.73 | 11 | 52.55 | 11.72 | |||
| 12 | 26 | 43.58 | 9.45 | 5 | 50.20 | 10.71 | 7 | 54.00 | 11.46 | |||
| Externalizing problem scoree | –0.224* | –0.107 | 0.338* | |||||||||
| 0 | 34 | 46.94 | 8.78 | 28 | 48.43 | 9.69 | 15 | 52.80 | 12.98 | |||
| 3 | 30 | 46.60 | 11.22 | 26 | 47.58 | 9.46 | 14 | 52.14 | 14.33 | |||
| 6 | 28 | 46.04 | 8.92 | 21 | 46.52 | 10.22 | 13 | 53.38 | 11.83 | |||
| 9 | 27 | 45.04 | 9.99 | 19 | 46.26 | 7.95 | 11 | 53.00 | 10.83 | |||
| 12 | 26 | 45.35 | 9.61 | 5 | 50.60 | 13.28 | 7 | 50.43 | 9.31 | |||
| Children's Global Assessment Scale score | 0.345** | 0.154 | 0.169 | |||||||||
| 0 | 32 | 75.56 | 9.85 | 27 | 72.78 | 11.38 | 13 | 70.85 | 13.30 | |||
| 3 | 30 | 77.87 | 11.32 | 24 | 72.88 | 10.93 | 14 | 70.07 | 12.97 | |||
| 6 | 27 | 76.78 | 10.56 | 20 | 73.90 | 11.54 | 14 | 70.93 | 12.02 | |||
| 9 | 27 | 79.33 | 9.75 | 19 | 74.68 | 12.65 | 10 | 68.40 | 10.66 | |||
| 12 | 25 | 79.24 | 10.09 | 5 | 74.60 | 13.35 | 7 | 74.00 | 8.16 | |||
Children Receiving Treatment
Discussion
Footnotes
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

