Behavior and personality characteristics differ among patients with eating disorders, depending on subtype. Patients with binge eating or purging behaviors, such as anorexia nervosa, binge eating/purging type, and/or bulimia nervosa, often display impulsive and disinhibited personality characteristics. In contrast, those with anorexia nervosa, restricting type (
1–3), often show a restrictive and overly controlled behavioral style.
Disinhibition and impulsivity related to eating behaviors are hallmarks of bulimia nervosa (
4,
5). In recognition of this core feature of the disorder, the DSM-IV diagnostic description of bulimia nervosa incorporates the requirement that binge eating episodes include this disinhibited (out of control) characteristic (
6). Impulsivity may extend into other areas of life in addition to binge eating or purging (
2). For example, individuals with bulimia nervosa often report alcohol and drug abuse, self-harm, sexual disinhibition, and shoplifting (
5). Some data suggest that the basis of cognitive and behavioral disinhibition in bulimia nervosa may be related to serotonin dysregulation (
7), while neuropsychological studies have found evidence of disinhibition at a neurocognitive level in affected individuals. For example, relative to healthy comparison subjects, individuals with bulimia nervosa who used laxatives were observed to make more errors of commission on a go/no-go task and endorse higher ratings for impulsive behaviors on a self-report assessment (
4). Similarly, cognitive research in inhibitory processing using a motor stop-signal paradigm and a motor Stroop task found that patients with restricting type anorexia displayed superior response inhibition overall, with fewer impulsive errors, than patients with the binge eating/purging type (
8). Anorexia nervosa patients with binge eating/purging behaviors also made more response errors on a modified version of the Hayling sentence completion task relative to patients with the restricting subtype (
8,
9). Thus, because of the evidence suggesting similarities in impulsive cognitive styles between anorexia nervosa, binge eating/purging type, and bulimia nervosa, we grouped both disorders together as a single category of binge eating/purging-type eating disorders in order to compare these patients with individuals in a restricting type anorexia group and a healthy comparison group for this study of cognitive inhibitory control.
Neural differences in executive functioning related to inhibitory control may be associated with the cognitive and clinical symptoms in bulimia nervosa (
10–12). However, only limited functional imaging studies have examined inhibition and disinhibition in bulimia nervosa to date. A recent study conducted by Marsh et al. (
11) examined response inhibition in adult patients with bulimia nervosa. These authors found that adult subjects with bulimia nervosa responded more impulsively and made more errors on a response inhibition task (Simon task [13]) relative to healthy comparison subjects, and patients with the most severe symptoms made the most errors. During correct responding on incongruent trials, patients failed to activate frontostriatal circuits to the same degree as healthy comparison subjects, including the bilateral inferior frontal gyrus, lenticular and caudate nuclei, and the anterior cingulate cortex. Marsh et al. concluded that diminished activity in these regions may contribute to the loss of control in the eating behavior of patients with bulimia nervosa. In contrast to patients with bulimia nervosa, clinical reports document perseverative, obsessive, and rigid thinking styles in patients with anorexia nervosa. Patients with anorexia nervosa are frequently perfectionistic and report obsessive-compulsive personality traits and obsessive-compulsive disorder (OCD) in childhood (
1). Studies have also found that those who have recovered from anorexia nervosa continue to exhibit anxiety, perfectionism, inflexible thinking, and overconcern with symmetry, exactness, and order (
14,
15). Neuropsychological research provides additional evidence of reduced cognitive flexibility (set shifting) and an excessively detailed information processing style (weak central coherence), with a neglect of the overall picture (gestalt), in adults with anorexia nervosa (
9,
12,
16,
17). Limited neuroimaging data in adults with anorexia nervosa support this idea as well. For example, Zastrow et al. (
12) found decreased activation in the anterior cingulate cortex and striatum associated with impaired cognitive behavioral flexibility.
Adolescents with eating disorders have rarely been examined in structural or functional neuroimaging studies (
18). It is well known that brain development undergoes significant alteration in adolescence (
19), and development of executive functioning skills is a particularly dynamic process during this period (
20), associated with increasing abilities pertaining to decision making, social processing, and inhibitory control. These refinements lead to what has been called the collaborative brain (
21), wherein improved connections allow the prefrontal cortex to modulate critical interconnected subcortical structures (e.g., the basal ganglia and amygdala). The lateral prefrontal cortex has a distinct architectonic “trend” within the frontal lobe (
22). The lateral aspect develops later than other regions, both in ontogeny and in phylogeny, and is important for making inhibitory processes more efficient.
In summary, phenomenological, clinical, neuropsychological, neuroimaging, and neurodevelopmental findings support the likely importance of examining inhibition/disinhibition in adolescents with eating disorders. Further, evidence suggests that aberrant functioning of the inferior frontal and anterior cingulate gyri is likely to be associated with impulsivity and disinhibition in anorexia nervosa patients who binge eat and purge but not in those with the restricting subtype (
11). Examination of response inhibition using functional magnetic resonance imaging (fMRI) in adolescents with eating disorders, specifically comparing those with restricting type anorexia with those who have bulimia nervosa or anorexia nervosa, binge eating/purging type, provides an opportunity to explore these processes in the developing brain and to distinguish patients with these subtypes from each other on a neural basis as well as from healthy comparison subjects. Examining neural correlates of inhibitory control in an adolescent population that is not severely emaciated and not chronically ill may help to distinguish these features associated with the onset of eating disorders as opposed to the secondary effects associated with starvation and prolonged disease.
This preliminary study is the first, to our knowledge, to examine brain activation associated with response inhibition in adolescents with eating disorders and, further, to compare patients with binge eating/purging behaviors with patients with a restrictive subtype of anorexia nervosa and with a healthy comparison group. We hypothesized that brain activation associated with inhibitory control during a go/no-go task would differ in adolescents with eating disorders relative to a healthy comparison group. We predicted that those with binge eating/purging behaviors would have abnormal activation in frontostriatal regions typically associated with response inhibition relative to healthy comparison subjects as well as to patients with anorexia nervosa, restricting type. We also predicted that we would find evidence of excessive inhibition in the restricting type anorexia group relative to both healthy comparison subjects and patients with binge eating/purging behaviors.
Method
This study was approved by the Stanford University Internal Review Board, and all volunteers signed informed consent (signed by parents for participants <18 years old) and/or assent (for participants <18 years old) forms before participation. Eating disorder subjects were current outpatients recruited from the Stanford University Child and Adolescent Psychiatry Clinic. Diagnoses of eating disorders and comorbid psychiatric disorders were made by clinicians with expertise in eating disorders in children and adolescents, and diagnoses were confirmed by the Eating Disorder Examination (
23), which was administered independently by trained interviewers. Participants with eating disorders were required to meet full DSM-IV criteria for their respective diagnosis within 3 months of study participation. Subjects with eating disorders also completed other diagnostic and clinical assessments (
Table 1). Volunteers were 16 female subjects with anorexia nervosa, restricting type; 15 with binge eating/purging behaviors (11 with bulimia nervosa and four with anorexia nervosa, binge eating/purging type); and 16 healthy comparison subjects. None of the subjects with bulimia nervosa had a history of anorexia nervosa, and none of the subjects with restricting type anorexia had a history of bulimia nervosa. Healthy comparison subjects were recruited through advertisements in local newspapers. For all three groups, eligible participants had no contraindications for magnetic resonance imaging (MRI) and no coexisting major neurological problems (e.g., seizure disorder, traumatic brain injury with loss of consciousness, multiple sclerosis) or psychotic disorders.
MRI Acquisition
Imaging data were acquired on a 3.0 Tesla GE Signa Excite magnet (General Electric Co., Milwaukee), housed in the Lucas Imaging Center of Stanford University, using a custom-built whole head coil providing a 35% advantage in signal-to-noise ratio over that of the standard GE coil. Following a three-plane localizer scan, fMRI data were collected using a spiral-in/-out sequence, which provides optimal signal-to-noise while minimizing susceptibility effects (
24). Thirty axial slices (3 mm thick, 1 mm skip) parallel to the anterior commissure-posterior commissure line and covering the whole brain were imaged (TR=2,000 msec; TE=30 msec; flip angle=90°; 1 interleave; field of view=22 cm
2; matrix=64×64; in-plane spatial resolution=3.125 mm). The task was presented using E-Prime software (Psychology Software Tools, Pittsburgh), which also triggered the initiation of the scan. Visual stimuli were projected from the foot of the scanner onto a screen attached to the head coil and viewed via a mirror.
Subjects performed a rapid, jittered event-related go/no-go task featuring a series of letters. They pushed a button in response to all letters, except for the infrequent letter X. This task is a classic test of executive function, requiring effortful inhibition of a prepotent response (
25). The task lasted 16 minutes and displayed 300 go stimuli and 75 no-go stimuli (1:4 ratio). The intertrial interval was jittered from 2 to 12 seconds.
Data Analysis
Functional data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London). Functional MRI data were corrected for slice timing, spatially realigned, motion repaired using the ArtRepair toolbox (
http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm), normalized into an age-appropriate stereotactic template from the Cincinnati Children's Hospital Medical Center (
https://irc.cchmc.org/software/pedbrain.php), smoothed with a 7-mm Gaussian filter, and high-pass filtered. A fixed-effects model compared correctly inhibited no-go trials with correct go trials for each subject.
Within-group t tests were performed using random-effects analyses and a significance threshold of p=0.01 height and p=0.01 extent, corrected for multiple comparisons. For all SPM5 analyses, the location of significant clusters and the coordinates of peak voxels in Talairach space were determined using the mni2tal function (
http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
The three subject groups were compared using a two-step approach. First, a whole-brain analysis of variance (ANOVA) was performed in SPM5 in all three groups for the contrast of correct no-go versus go trials. Significant clusters of activation associated with the main effect of group were identified using a threshold of p=0.01 height and extent, equalling 80 voxels, uncorrected. Although this threshold is uncorrected, it is more stringent than that used in many fMRI studies and is appropriate for the initial step of this analysis identifying clusters of interest. In the second part of the analysis, the mean activation in each of the identified clusters was extracted using the MarsBaR toolbox (
http://marsbar.sourceforge.net/) and transferred to SPSS (SPSS, Inc., Chicago) for follow-up t tests. The t tests, conducted in SPSS, included age as a covariate (because of group differences in age) and used a corrected significance threshold of 0.0083. This threshold was determined as a p value of 0.05 divided by the number of clusters identified in the SPM5 whole-brain analysis (see Results section). Limiting the follow-up t tests to regions of interest identified by the three-group ANOVA reduced the probability of type I error. Extracting these regions of interest into SPSS allowed for correlation analyses with clinical and behavioral variables and examination of distributions associated with each group. Behavioral and demographic data were also analyzed in SPSS using ANOVA and follow-up t tests when appropriate.
Whole brain correlations with task accuracy were conducted in SPM5 using multiple regression. A threshold of p=0.01 height and p=0.01 extent, corrected for multiple comparisons, was used to determine significance.
Correlations between clinical and behavioral measures and brain activation in the extracted regions of interest were performed in SPSS using a Pearson's correlation analysis. The most relevant clinical measures were chosen for analysis, including total scores on the Eating Disorder Examination, Beck Depression Inventory (BDI), Behavioral Inhibition Scale, and Multidimensional Anxiety Scale for Children. A corrected alpha of 0.0125 (0.05/4 [four clinical measures]) was chosen as a significance threshold.
Results
Two subjects with anorexia nervosa, restricting type, two with binge eating/purging behaviors, and three healthy comparison subjects were removed from the data analyses, either for excessive motion in the scan or for behavioral data with <50% correct responses, which suggested noncompliance with or misunderstanding of task instructions. The remaining subjects were 14 with anorexia nervosa, restricting type, 13 with binge eating/purging behaviors, and 13 individuals in the healthy comparison group.
Table 1 shows the demographic and clinical characteristics of each group. Handedness did not differ between groups. Age, scores on the Eating Disorder Examination, and behavioral symptom reports are consistent with the clinical presentation of these disorders in adolescents. Therefore, participants with bulimia nervosa and anorexia nervosa, binge eating/purging type, were at higher weights and were slightly older than participants with anorexia nervosa, restricting type (
26). There was a significant difference in age across the three groups, primarily associated with a low variance in age within each group (F=7.03, df=, 2, 37, p=0.003). Patients with binge eating/purging type were older than those with the restricting subtype and individuals in the healthy comparison group (binge eating/purging type: mean age=17.26 years [SD=1.18]; healthy comparison: mean age=15.93 years [SD=1.33]; anorexia nervosa, restricting type: mean age=15.02 years [SD=1.74]). Accordingly, we performed between-group comparisons of functional activation in SPSS with and without age as a covariate. Further analyses of the effects of age on brain activation are included in this report.
Behavioral Performance
All participants performed with high accuracy on the go trials and had a similar rate of false alarms on the no-go trials. The mean accuracy (percent correct) for go trials in the healthy comparison group was 94.64% (SD=9.96); in the binge eating/purging group it was 97.38% (SD=2.65); and in the restricting type anorexia group it was 97.71% (SD=3.31). The mean response time for go trials in the healthy comparison group was 386.49 msec (SD=55.88); in the binge eating/purging group it was 403.46 msec (SD=45.05); and in the restricting type anorexia group it was 356.54 msec (SD=90.68). The mean accuracy for no-go trials in the healthy comparison group was 73.74% (SD=14.54); in the binge eating/purging group it was 77.23% (SD=10.77); and in the restricting type anorexia group it was 70.86% (SD=12.11). The mean response time for false alarms in the healthy comparison group was 350.07 msec (SD=43.74); in the binge eating/purging group it was 333.09 msec (SD=29.23); and in the restricting type anorexia group it was 341.62 msec (SD=25.13). There were no group differences in task accuracy for go or no-go trials. Additionally, there were no group differences in response time for go trials.
Correlations between age and task performance were not significant across groups. Within groups, only one correlation was marginally significant, which was within the restricting type anorexia group, where age was correlated with response time for false alarms (r=0.54, p=0.047). Therefore, although older subjects took longer to make a false alarm on a no-go trial, the response time for false alarms was not significantly different between groups.
Neuroimaging Findings
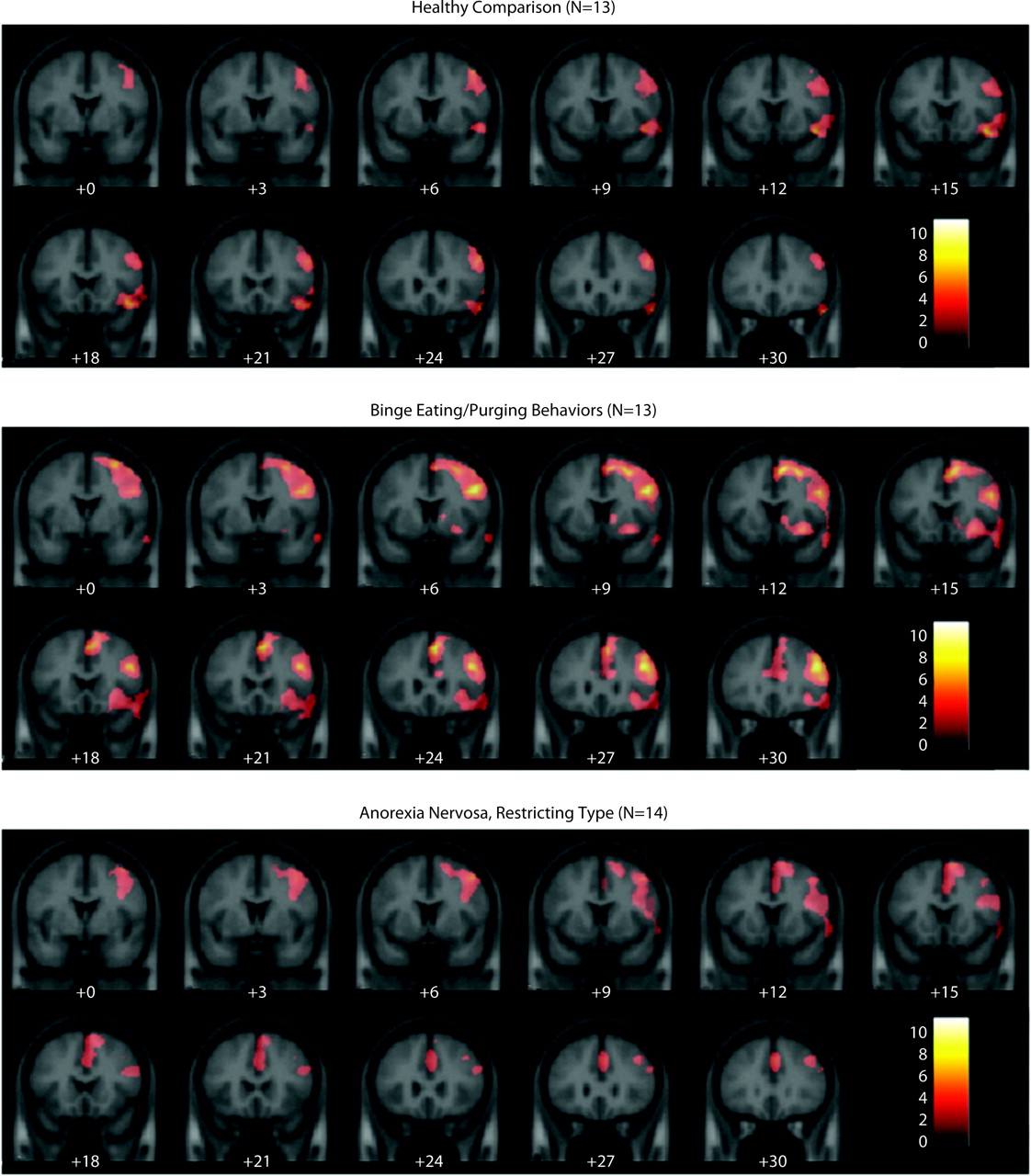
Functional MRI results showed that within each group, the right inferior and middle frontal gyri were activated during successful response inhibition trials (i.e., not pressing when observing the letter X) compared with successful response trials (no-go/go) (
Figure 1,
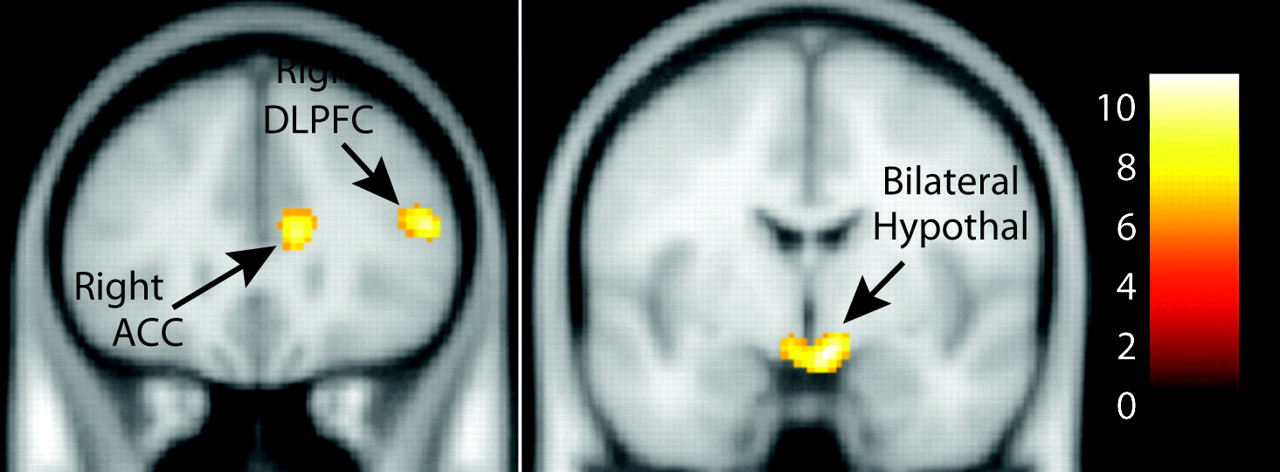
Table 2). Additionally, patients with binge eating/purging behaviors showed right dorsal caudate and anterior cingulate cortex activation as well as widespread frontal activation. Patients with restricting type anorexia also showed anterior cingulate cortex activation but not caudate activation. A three-group ANOVA showed a significant main effect of group in the bilateral hypothalamus, right dorsolateral prefrontal cortex, right anterior cingulate cortex, right middle temporal gyrus, and bilateral precentral gyri (
Figure 2,
Table 3). Follow-up between-group comparisons performed in SPSS (corrected p=0.0083) showed that all of the group differences were attributed to increased activation in the binge eating/purging group versus the healthy comparison group and versus the restricting type anorexia patients (left precentral gyrus: p=0.001 and p=0.011 [nearly significant], respectively; right precentral gyrus: p=0.0001 and p=0.006; right anterior cingulate cortex: p=0.00001 and p=0.013 [nearly significant]; right middle/superior temporal gyrus: p=0.0001 and p=0.005; hypothalamus: p=0.001 and p=0.001; right dorsolateral prefrontal cortex: p=0.001 and p=0.001). When the analysis was repeated using age as a covariate, all of the group differences between patients with binge eating/purging behaviors and healthy comparison subjects remained. Greater activation in the binge eating/purging group compared with the restricting type anorexia group remained only in the hypothalamus and the dorsolateral prefrontal cortex (
Table 3). As mentioned previously, a corrected alpha of 0.0083 was used for significance (p=0.05/6 [six regions]).
Although the analyses were performed while covarying for the group difference in age, we further tested whether age had an effect on activation in any of the regions of interest. We conducted Pearson's correlations between activation in each brain region and age, both within each group and across all groups. None of the correlations were significant (at a threshold of 0.05), suggesting that age did not significantly influence our results.
The low rate of false alarms greatly reduced our ability to detect brain activation associated with false alarm trials. Typically, approximately 30 trials are needed to detect activation in an event-related study, corresponding to a false alarm rate of 40% (out of a possible 75 no-go trials). Only three comparison subjects and four restricting type anorexia patients made ≥40% false alarms, while no binge eating/purging patients made ≥40% false alarms. Instead, we performed a whole-brain correlation with the percent of correctly inhibited trials within each group, using a significance threshold of p=0.01 height and p=0.01 cluster extent, corrected for multiple comparisons. Therefore, this analysis suggests group-specific neural strategies that are successful (positive correlation) or unsuccessful (negative correlation). We found that only restricting type anorexia patients showed a significant positive correlation with the percent of correctly inhibited trials in a single large cluster (cluster size=3,657 voxels; peak: Z=3.72; location: x=18, y=−80, z=28). The cluster included regions of the inferior parietal cortex (Brodmann's area 7), precuneus (Brodmann's area 19/31), and posterior cingulate gyrus (Brodmann's area 31). This suggests that within the restricting type anorexia group, successful inhibition is associated with greater recruitment of brain regions underlying visual attention (
27) and visual working memory (
27) (e.g., the precuneus and inferior parietal cortex). Attention to detail is a cognitive feature associated with anorexia nervosa (
17).
Association With Clinical Measures
Pearson's correlation analysis was used to find associations between activation in the extracted regions of interest and relevant clinical measures, including scores on the Eating Disorder Examination, BDI, Behavioral Inhibition Scale, and Multidimensional Anxiety Scale for Children. A threshold of 0.0125 (0.05/4 [four clinical measures]) was required for significance. Across all groups combined, the total Eating Disorder Examination score was significantly correlated with activation in the bilateral precentral gyrus, right anterior cingulate cortex, right superior temporal gyrus, and hypothalamus. However, these correlations were attributable to group differences in the score. The correlations were repeated within each group separately and were not significant. In addition, there were no significant correlations with subscales on the Eating Disorder Examination or the number of bulimic episodes. Similarly, for BDI measures, significant correlations across all groups were found with the bilateral precentral gyrus and right superior temporal gyrus, but these were because of group differences in scores. Correlations between BDI scores and region of interest values were not significant within each group separately. Scores on the Behavioral Inhibition Scale were not correlated with activation in any of the regions of interest. The Multidimensional Anxiety Scale for Children was significantly correlated with right superior temporal gyrus activation across all groups (r=0.48, p=0.002). This was found to be attributable primarily to a significant correlation between scores on this measure and right superior temporal gyrus activation within the binge eating/purging group (r=0.70, p=0.007) but was not significant within the other groups.
Possible effects of weight restoration on outcome were examined. In the binge eating/purging group, only two subjects weighed <86% of their ideal body weight. However, to determine whether this variable affected our results, we excluded the two low-weight subjects in this group and reran the comparisons with the healthy subjects. The results did not change for any of the six brain regions, even while covarying for age. Therefore, low weight did not appear to influence the comparisons between subjects in the binge eating/purging and healthy comparison groups. Comparisons with the restricting type anorexia group were not possible, since six of the 14 subjects were <86% of their ideal body weight, although none were extremely emaciated (e.g., <79% of the ideal body weight), as all were outpatients. Because hormonal function is related to nutritional status, we conducted an analysis excluding the single participant in the restricting type anorexia group who reported regular menstruation at the time of scanning and found no difference in activation patterns.
Because scores on the BDI were significantly higher for the binge eating/purging group, we examined the possible influence of major depression on our findings by removing from the analysis the three subjects who had a diagnosis of major depression. These were two subjects in the binge eating/purging group and one subject with anorexia nervosa, restricting type. None of the results changed. For all of the regions of interest, activation in the binge eating/purging group remained significantly greater than in the healthy comparison group (range for significance: 0.002–0.0080, including covariance for age).
Discussion
This preliminary study supports the hypothesis that differences in neural function can be identified between anorexia nervosa, restricting type, and anorexia nervosa, binge eating/purging type, as well as between individuals with binge eating/purging behaviors and healthy comparison subjects during a task requiring inhibitory control. Patients with the binge-purge subtype showed increased activation in the right dorsolateral prefrontal cortex, an executive control region, suggesting inefficient or possibly compensatory activation (i.e., recruitment of additional brain regions and/or discrepant brain activation patterns leading to improved cognitive ability) (
28). The finding of increased hypothalamic activation further suggests aberrant responses in a region associated with emotional function (
29,
30). This finding might also indicate that the binge eating/purging behaviors group experienced greater stress during response inhibition (
31), possibly related to the additional effort needed to successfully complete the task. Binge eating/purging subjects use greater anterior cingulate cortex resources as well, suggesting increased activity related to monitoring response conflict (
32). This finding differs from that identified by Marsh et al. (
11) and Uher et al. (
33), who reported decreased activation in the frontostriatal region in adults with bulimia nervosa. Additionally, Marsh et al. found that adult patients with bulimia nervosa had impaired task performance, while our study did not. These differences could result from task differences (Simon task [13])—a response inhibition task with incongruent spatial stimuli that may be more challenging than the go/no-go task [34, 35])—developmental differences (age, cognitive maturity), clinical severity or chronicity (duration of illness, binge eating/purging behavior frequency), diagnosis (binge eating/purging behavior versus bulimia nervosa), or comorbidity. The fact that our younger age group activated several additional brain regions not observed in the Marsh et al. study (e.g., hypothalamus, dorsolateral prefrontal cortex) might also reflect the influence of brain maturation on cognitive processing.
In a whole-brain correlation with the percent correct on no-go trials within each group, only patients with restricting type anorexia showed a significant positive correlation with activation in a cluster that included the inferior parietal cortex, precuneus, and posterior cingulate gyrus. The other groups showed no significant correlations with the percent correct on no-go trials.
Clinically, adolescents with restricting type anorexia display characteristics of greater inhibitory control than healthy comparison subjects and those with bulimia nervosa or anorexia nervosa, binge eating/purging type, while subjects with bulimia nervosa or binge eating/purging type anorexia display decreased inhibitory control relative to healthy comparison subjects and those with restricting type anorexia. Our findings show putative neural correlates of decreased inhibitory control in female adolescents who binge eat and purge, but we did not find evidence of comparable correlates of increased inhibitory control in patients with anorexia nervosa, restricting type. The neurofunctional correlates of these cognitive characteristics in binge eating/purging subjects are consistent with expected locations of activation related to executive function (
18,
30,
33). However, there were no correlations between Eating Disorder Examination subscale scores or binge-purge episodes, but this may be a result of poor reliability of the subscales and low variability of the rates of binge eating and purging in this small sample. This could also imply that severity of behavioral symptoms (e.g., binge-purge rates) do not predict activation within this group.
Our preliminary findings suggest that adolescent subjects with binge eating/purging behaviors and restricting type anorexia likely differ from each other on a neural level, and therefore risks and effective interventions may differ between these two groups. These findings in an adolescent group not severely malnourished and with a relatively short duration of eating disorder symptoms suggest that these neural processes occur prior to or early in the evolution of the disorder and may not be the result of chronic disease or state-dependent starvation. Longitudinal studies are needed to help distinguish primary and secondary neural processes. Other studies might also follow individuals with early symptoms of an eating disorder to track the development of behaviors and corresponding neural correlates. In addition, future studies might also examine the effects of cognitive therapies on these neural processes in subjects with restricting type anorexia and those with binge eating/purging behavior (
36).
There are a number of limitations to these findings. The sample is small, and therefore other group differences may well have not been detected. The participants were female patients, and although most individuals with eating disorders are female patients, 10% are male patients, and these findings may not generalize to a male population. The go/no-go task does not capture all aspects of inhibitory control. Additional studies are needed to replicate and expand upon these preliminary findings. It is also important to note that depression symptoms are associated with some of the regions of interest examined in this study (
37,
38). Depression is a common comorbid condition in bulimia nervosa, and BDI scores were elevated in the binge eating/purging group. However, there was no correlation found between BDI scores and any of the regions of interest within this group in our study.
An ongoing debate in the diagnostic literature is the potential crossover between anorexia nervosa, restricting type; anorexia nervosa, binge eating/purging type; and bulimia nervosa, particularly whether eating disorders are best considered a single transdiagnostic disorder or separate clinical entities (
39). The present study provides preliminary evidence that at least during adolescence, eating disorder subtypes may be distinguishable in terms of neural correlates of inhibitory control. This distinction is also consistent with clinical reports of the later onset of binge eating and purging in general and in anorexia nervosa, binge eating/purging type, in particular (
26). At the same time, the fronto-striatal circuit is known to be involved in a variety of psychiatric disorders (e.g., Tourette's syndrome, bipolar disorder, OCD, attention deficit hyperactivity disorder, depression). Thus, these findings should be considered in this larger context (
18). Addressing inhibitory control as an aspect of treatment in adolescents with eating disorders is another important consideration. Strategies to address response inhibition, as well as cognitive flexibility and perseverative thinking, using cognitive remediation therapy have been preliminarily studied in adults with chronic anorexia nervosa and may be a promising adjunctive treatment to standard interventions aimed at weight restoration and eating disorder-related cognitions (
36).