Autism spectrum disorders are considered to be among the most heritable of all mental disorders. For example, recent reviews estimate the heritability of autistic disorder to be more than 90% (
1,
2). This figure has gained wide acceptance despite the fact that it is based on surprisingly sparse data— essentially one British twin study of 21 twin pairs (
3,
4), later extended with 26 additional pairs (
5), and one Nordic population-based study of 22 twin pairs (
6), using wide age ranges to identify a sufficient number of affected twin pairs. Heritability is defined as the proportion of variation in the total population that is due to genetic variation. Deriving heritability estimates from clinically ascertained samples is not straightforward (
7) (as exemplified by the heritability estimate from the British study, which gave two different heritability estimates depending on the [unknown] population prevalence [5]) and may differ from estimates derived from population-based samples. Only a few studies have used more recent concepts of autism spectrum disorders, with similar results (
8,
9). Thus, the heritabilities of both narrowly defined autistic disorder and autism spectrum disorders are still not well documented.
Another line of research has instead tried to study autistic traits in the general population. In a U.K. twin sample, autistic traits in a general population of 3,500 8-year-old twin pairs were highly heritable (≈80%) both as a continuous trait and when studied at the quantitative extreme (
10). Smaller twin studies have shown significant genetic effects (albeit with limited precision) on traits of social pervasive developmental problems from the Child Behavior Checklist (
11), the Social Responsiveness Scale (
12), and the Autism-Spectrum Quotient (
13).
Similar problems with identifying the magnitude of genetic influences are encountered in other childhood-onset neuropsychiatric disorders, with the possible exception of attention deficit hyperactivity disorder (ADHD), for which twin studies have estimated the heritability to be 60%–80% (
14). Population-based information on the heritability of developmental coordination disorder is still lacking, and both genes and shared environments have been implicated in the familial aggregation of tic disorder in childhood (
15,
16).
Various child psychiatric disorders are traditionally considered to be separate conditions. Nevertheless, these disorders have a high degree of overlap, and “pure“ cases are rare in both clinical and population-based studies. Virtually every individual with an autism spectrum disorder displays some form of comorbidity (
17,
18). Similarly, a substantial number of children with ADHD have autistic traits (
19,
20). Also at a subclinical level, twin studies suggest that autistic traits are linked to ADHD symptoms and that this association is due to a common genetic etiology (
21,
22).
In the current genomic era when causal genes can be identified, the value of quantitative genetic modeling might be questioned. However, the identification of genes that are important in psychiatric disorders has been much harder than expected. The large International Schizophrenia Consortium, with about 7,000 case and control subjects, was unable to identify single-nucleotide polymorphisms (SNPs) that reached a genome-wide significance level of 10
−8. After combining with other consortia and thus using 8,008 case subjects and 19,077 control subjects, the best hit (in the major histocompatibility complex region) yielded a decreased risk for schizophrenia for the minor allele of about 20%—explaining a minuscule part of the risk variance. Instead, the study provided molecular genetic evidence for a substantial polygenic component to the risk of schizophrenia involving thousands of common alleles of very small effect (
23). Thus, it is doubtful whether molecular genetics will identify a large fraction of the genes contributing to susceptibility to psychiatric disorder, and identification of susceptible genes will be an even larger challenge when studying comorbidity (
24). On the other hand, twin studies, which examine the sum of all susceptibility alleles and therefore investigate the effect of all genes collectively, constitute a powerful tool for clarifying common pathophysiological pathways and for answering fundamental questions about whether two different psychiatric disorders are related genetically.
We interviewed the parents of all 9-year-old and 12-year-old twins in Sweden during a 5-year period, focusing particularly on autism and related neuropsychiatric disorders. This enabled us 1) to calculate population-based heritability estimates for autism spectrum disorders, ADHD, developmental coordination disorder, and tic disorder, and 2) to investigate whether genetic effects are responsible for the overlap among the different neuropsychiatric conditions.
Method
Subjects
The parents of all 21,790 Swedish 9-year-old (born in July 1995 or later) and 12-year-old (born July 1992-June 1995) twins were identified through the Swedish Twin Registry and contacted for interviews by telephone as part of the Child and Adolescent Twin Study in Sweden (
25). The reason for choosing this age group was that most of the major child psychiatric problem constellations have been established by then, whereas the complex biopsychosocial problems associated with puberty have not yet emerged.
Interviewers from a professional company (Intervjubolaget, Stockholm) carried out the interviews after receiving a brief introduction to child and adolescent psychiatry and twin research. The study started in July 2004 and remains under way. As of November 2009, 80% of the parents of the cohorts born before May 2000 had responded. The mother was interviewed in 88% of cases and the father in 12%; in 30 cases (0.4%) another member of the family was interviewed.
The total sample consisted of 17,036 individuals. Of these, 156 had documented brain damage (most commonly cerebral palsy) and 22 had a known genetic syndrome (most commonly Down's syndrome but also fragile X syndrome) and were therefore excluded. The analyses were performed on the remaining 16,858 twins.
Zygosity Determination
Zygosity determination for 571 pairs of twins for whom we had DNA on both twins was based on a panel of 48 SNPs. For the remaining twins, we used an algorithm based on five items concerning twin similarity and confusion derived from the twins with known zygosity. Only twins with more than a 95% probability of being correctly classified were assigned a zygosity. Using this algorithm we analyzed 1,104 monozygotic male twin pairs, 1,543 dizygotic male twin pairs, 1,138 monozygotic female pairs, 1,356 dizygotic female pairs, and 2,841 opposite-sexed dizygotic pairs.
Measures
Child neuropsychiatric problems were identified using the Autism-Tics, ADHD, and Other Comorbidities inventory (A-TAC), a comprehensive parent interview focusing on child autism spectrum disorders and associated conditions that has been validated for administration by laypersons over the telephone (
26,
27). The instrument includes questions covering 96 specific child psychiatric problems, organized in modules theoretically defined in accordance with established diagnostic categories. Each module is assessed separately without diagnostic hierarchies. Questions are answered in a lifetime perspective and in relation to age peers. Three response categories are used: “no” (coded 0), “yes, to some extent” (coded 0.5), and “yes” (coded 1.0).
Test-retest reliabilities and interrater reliability for autism spectrum disorders, ADHD, developmental coordination disorder, tic disorder, and learning disorders were good to excellent (intraclass correlations of 0.87 or above), and an early validation study showed good sensitivity but lower specificities (
26). To improve the possibility of assigning research proxies for clinical diagnoses, the scales were modified and again validated with interviews with parents awaiting clinical neuropsychiatric investigations (
27). For the 17 items on autism spectrum disorders, a score ≥8.5 yielded a specificity of 0.95 for identifying proxies to clinical diagnoses defined according to DSM-IV criteria for a pervasive developmental disorder. The validation studies have not supported discriminatory validity between the subtypes, such as Asperger's disorder, “classic” autism, or atypical autism. For ADHD (19 items), the corresponding cutoff was 12.5 (specificity, 0.95); for developmental coordination disorder (1 item), 1.0 (specificity, 0.95); for tic disorder (3 items), 1.5 (specificity, 0.90); and for learning disorders (3 items), 3.0 (specificity, 0.96) (
31). Sensitivities for these cutoffs were 0.71 for autism spectrum disorders, 0.52 for ADHD, 0.28 for coordination disorder, 0.92 for tic disorder, and 0.23 for learning disorders.
When studying comorbidity, low prevalences of the disorders limit the statistical power to find significant differences between monozygotic and dizygotic pairs. We therefore performed sensitivity analyses with the lower cutoffs for diagnoses suggested in the validation study (
27). These cutoffs were 4.5 for autism spectrum disorders (sensitivity, 0.96; specificity, 0.88), 6.0 for ADHD (sensitivity, 0.98; specificity, 0.81), 0.5 for developmental coordination disorder (sensitivity, 0.59; specificity, 0.85), 1.5 for tic disorder (sensitivity, 0.92; specificity, 0.90), and 1.0 for learning disorders (sensitivity, 0.88; specificity, 0.75).
Analysis
The concordance rate (that is, the risk of a disorder for the co-twin of a twin with the same category of disorder) was calculated as the proportion of individuals belonging to concordantly affected twin pairs out of all twins with the disorder.
Quantitative genetic analyses were used to estimate the relative importance of hereditary and environmental factors for variation in liability to child neuropsychiatric problems (
28). Phenotypic variance was divided into one component due to inherited genetic factors (heritability), one component due to environmental factors common to both members of the twin pair (shared environmental component), and one component due to environmental factors unique to each twin (nonshared environmental component). Standard univariate analyses using structural equation modeling with all types of twins simultaneously were used to provide estimates of the unobserved variables, that is, additive genetic, shared environmental, and nonshared environmental effects (
28). In brief, the correlations between the genetic and environmental factors for the twins were set to their theoretical values (1 and 0.5 for additive genetic effects for monozygotic and dizygotic twins, respectively, as monozygotic twins share their entire genome whereas dizygotic twins share on average 50% of their segregating genes; 1 for shared environmental effects for both types of twins, as monozygotic and dizygotic twins share environments to the same degree).
The assumption of an underlying normal distribution of liability (or susceptibility) to the disorders was applied. We allowed for different threshold values for boys and girls.
When studying comorbidity, we used the bivariate Cholesky model in the structural equation model fitting (
28). The genetic correlation (r
g) parameter provides evidence for potential genetic comorbidity.
We used the statistical package Mx (
29). Because trying to identify best-fitting models with discrete twin data could lead to incorrect conclusions (
30), we only examined the full model here. Fit of models was evaluated by using the root mean squared error of approximation, which is better than the chi-square test when using large samples (
28,
31). Values less than 0.08 are generally considered to represent an acceptable model fit, and values less than 0.05 are indicative of a good fit.
Results
In total, 0.9% of the twins met criteria for autism spectrum disorders, 1.8% for ADHD, 1.6% for developmental coordination disorder, and 3.1% for tic disorder (
Table 1). Thirty-four percent of the children with autism spectrum disorders also had learning disorders. A high degree of overlap with learning disorders was also evident for the other neuropsychiatric disorders. Boys had significantly higher prevalences than girls for all disorders (p<0.001 for all disorders). There were no significant differences in prevalence between the 9-year-old and the 12-year-old children (data not shown).
Concordance rates for autism spectrum disorders for boys were 0.47 for monozygotic twins and 0.14 for dizygotic twins (
Table 2). Among girls, there was only one concordant twin pair. Monozygotic twins also had higher concordance rates than dizygotic twins for ADHD, developmental coordination disorder, and tic disorder.
The quantitative genetic models showed good fits for all four disorders (
Table 3; root mean squared error of approximation <0.05). Genetic effects (heritability) accounted for 80% (95% confidence interval [CI]=29−91) of the variation in liability for autism spectrum disorders (
Table 3). There was no indication of shared environmental effects. Rather, the remaining variation in liability was due to nonshared environmental effects. Genetic effects were also high and significant for ADHD, developmental coordination disorder, and tic disorder, again with little evidence for shared environmental effects.
Quantitative genetic analyses were performed for boys separately (see Table S1 in the data supplement that accompanies the online edition of this article); there was insufficient power to perform separate analyses for girls. The results were broadly similar to those presented in
Table 3.
Fifty-one percent of the children with an autism spectrum disorder also met criteria for ADHD (
Table 4). The probability of meeting ADHD criteria for a monozygotic co-twin of a twin with an autism spectrum disorder was nearly as high (44%), whereas the probability was 15% for a dizygotic co-twin (
Table 4). Results from formal model-fitting analyses suggested that genetic effects are important for the comorbidity; the genetic correlation was high (r
g=0.87, 95% CI=0.77−1.0) (
Table 4).
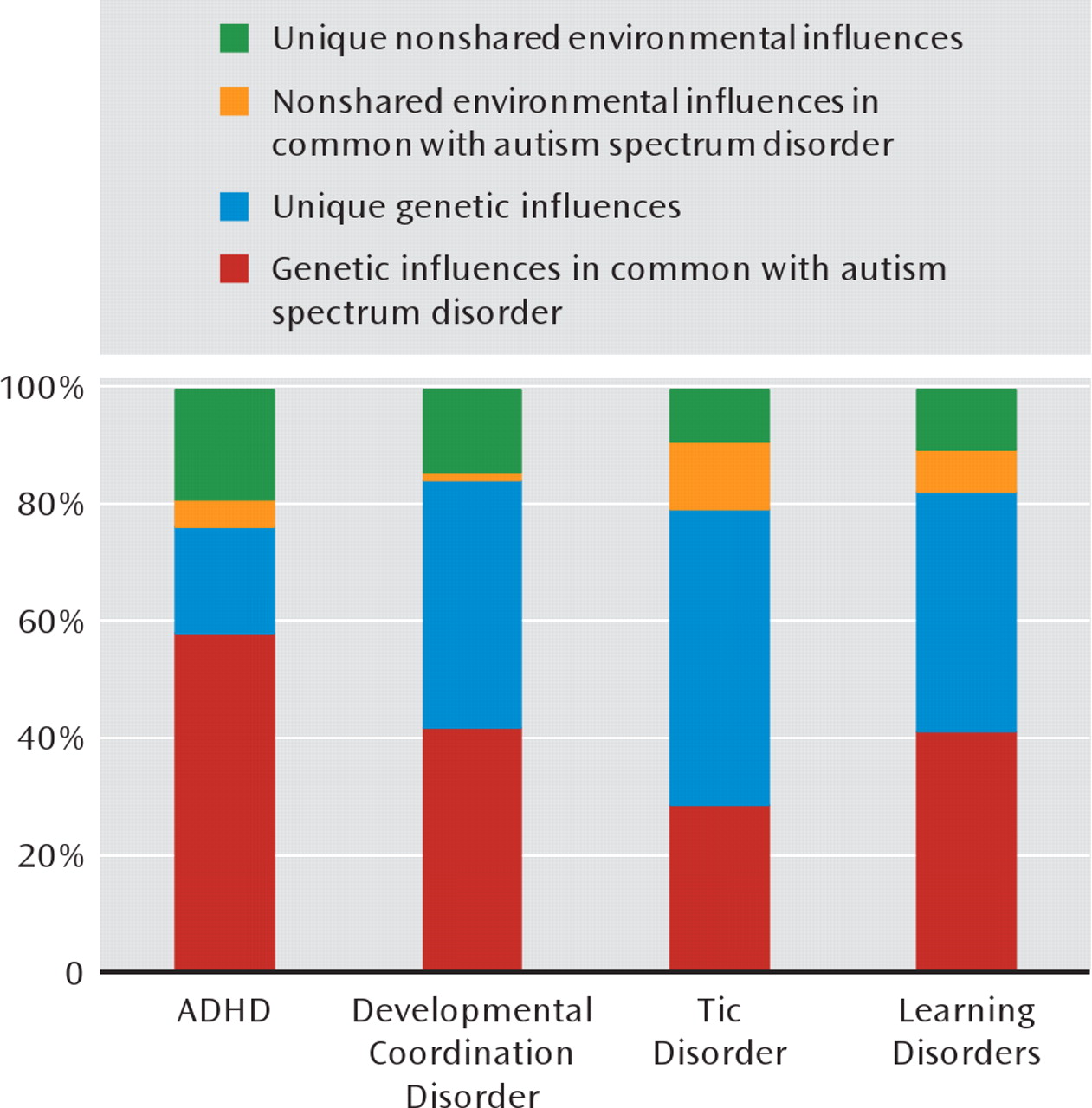
Figure 1 depicts the role of genetic and environmental influences in autism spectrum disorders in common with each of other neuropsychiatric disorders, along with the genetic and environmental variance components unique to autism spectrum disorders, as estimated from the bivariate model-fitting analyses. While about 80% of the variation in liability for autism spectrum disorders was due to genetic effects (
Table 3), the bivariate analyses suggest that about three-quarters of this genetic variance was actually shared with ADHD (
Figure 1).
There were also higher monozygotic than dizygotic cross-disorder effects for most other comorbidities (
Table 4). A substantial proportion of the genetic variance for autism spectrum disorders was shared with each of the other disorders, and the absolute majority of the coexistence of autism spectrum disorders with dysco-ordination, tics, and learning problems was due to a common genetic liability (
Figure 1). However, it should be noted that the low prevalence of the disorders led to large confidence intervals around the genetic correlation parameter estimates in
Table 4. Thus, even though these results provide strong evidence for genetic influences on comorbidities with all the neuropsychiatric disorders, the absolute values of the genetic correlations should be cautiously interpreted.
Because the main analyses had limited power, we performed sensitivity analyses with lower cutoffs for diagnoses, as suggested in the validation study (
27). The results of these sensitivity analyses confirmed the results of the main analyses. The estimates of the genetic correlations ranged from 0.29 to 0.88, with tighter confidence intervals (see Table S2 in the online data supplement).
Discussion
This is the largest population-based twin study ever performed of child neuropsychiatric disorders. Using parent interviews, we confirmed the high heritability for autism spectrum disorders (80%) suggested by previous studies of clinically referred samples (
3–6) as well as studies using more recent concepts of autism spectrum disorders (
8,
9). As hypothesized, a high comorbidity was found across the different neuropsychiatric disorders (including learning disorders), and the data suggest that genetic effects were of major importance for this comorbidity.
The concordance rate for autism spectrum disorders in boys was 0.47 for monozygotic and 0.14 for dizygotic twins. Concordance rates depend on the prevalence rates. The same difference in concordance between monozygotic and dizygotic twins could therefore yield different heritability estimates, and thus it is not meaningful to compare concordance rates between studies with different case definitions and prevalences. Therefore, we used structural equation models to estimate the relative importance of genetic and environmental effects and to compare with other studies. Our heritability estimate of 80% for autism spectrum disorders confirms the high heritability reported for more narrowly defined autistic disorder (
3–6). The broader phenotype of autism spectrum disorders reflects restrictions in social interaction, communication, and flexibility that range from severely disabling conditions to broader phenotypes. The definition of autism spectrum disorders used here does not distinguish between proposed–although continually questioned–subtypes. Thus, our study shows heritability estimates that are similar between the full autism spectrum and more narrowly defined phenotypes. Our estimate is also similar to estimates derived from studies of the entire distribution of autistic traits (
8,
12,
13,
32). Thus, the accumulated data suggest that caseness for an autism spectrum disorder, as well as subthreshold problems, represents the lowermost end of normal distributions of abilities in social communication and flexibility (
12).
The heritability estimate of 79% for ADHD is in agreement with previous studies using both categorical and continuous definitions of ADHD (
14,
33,
34). We also obtained a similar estimate for developmental coordination disorder (70%). As far as we know, no previous studies have investigated genetic effects on developmental coordination disorder. For tic disorder, the heritability was 56%. Previous studies of tic disorder have suggested that part of the familial aggregation might be ascribed to shared environmental effects. In a Japanese study of parents of twins, shared environmental effects accounted for about one-third of the variance in one question about “habitual behavior” (
15), and a U.K. study of 4- and 6-year-olds estimated the shared environmental effects at 11% for tic disorder (
16). Our study did not support the shared environmental effects for tic disorder; instead, the pattern was similar to the other neuropsychiatric disorders.
Our data provide evidence that genetic effects are important for comorbidity across the different neuropsychiatric conditions, including learning disorders. In general, the risk for a different neuropsychiatric diagnosis was higher in monozygotic than dizygotic twins, even though low prevalence decreased the power to detect significant genetic correlations. However, the analyses with lower thresholds supported this conclusion. In principle, all comorbidity was due to genetic effects, and a large part of the genetic susceptibility for autism spectrum disorders was shared with the other disorders (especially ADHD). These findings are consistent with previous twin studies (
11–13) and the concept of a general genetic susceptibility for developmental mental health problems, rather than a notion of specific genes as causes of specific disorders. Similar results suggesting a common genetic etiology for the main psychotic disorders (i.e., schizophrenia and bipolar disorder) as well as for personality disorders are now emerging, both from molecular genetic (
23,
35) and from genetic epidemiological (
36,
37) studies. It might be time to reevaluate the current psychiatric diagnostic entities among adults and children alike.
In this study, we screened 10,895 twin pairs—all twins born in Sweden between 1992 and 2000, with a very high response rate (>80%). However, the results should be interpreted in light of some limitations. Even with a nationwide cohort of this scale, the absolute numbers of screen-positive concordant or discordant pairs remain limited and comparable to previous clinical studies, even though an advantage of our design is that age and period effects are controlled for. Nevertheless, the confidence intervals are relatively large, and point estimates, especially for developmental coordination disorder and tic disorder, should be interpreted with caution. Statistical power issues also prohibited more elaborate twin analyses (such as investigations of dominant genetic variance). Since this epidemiological study included nearly 17,000 children, complete clinical evaluations were not feasible, and therefore we had to rely on parent interviews, which is not optimal. Clinical follow-up studies of children with neuropsychiatric disorders are urgently needed. We used an instrument (A-TAC) that has shown good psychometric properties, and for the disorders addressed in this study, thorough validation data are available (
26,
27). We also had to rely on parent reports to exclude cases of neuropsychiatric disorders due to medical conditions such as Down's syndrome, fragile X syndrome, and various forms of brain damage syndromes. As detailed above, about 1% of children were excluded because of information about such conditions. The prevalence of the genetic syndromes was surprisingly low. This was probably because parents with children with severe handicaps were less likely to participate in the study. Another area where we lack information is the cognitive level of the screen-positive children. Cognitive tests were not possible in this study design, so we had to rely on parents' information on special educational needs. This gives a high prevalence of learning disorders, a less skewed sex ratio, and less specific associations with other disorders than a test-based evaluation of mental retardation.
Twins may not be representative of the general population in terms of mental health problems and psychomotor development. It has been suggested that twins might have an increased risk for autism (
5), although this claim remains unsubstantiated. Nevertheless, as already discussed, this probably does not affect the interpretation of the genetic results (
5). Also, most empirical studies that have examined psychotic or general psychiatric outcomes (
38,
39) have found no or very small differences between twins and singletons. The coexistence of problems in this study may to some extent be explained by overall differences in response style by a single reporter. However, this is not a likely explanation of the genetic effects, because such bias should have led to similar effects in both monozygotic and dizygotic twins. Recall bias might have affected the results. However, there were very few significant differences between the 9-year-old and the 12-year-old children, supporting our view that the A-TAC generally succeeds in assessing symptoms and problems in a lifetime perspective.