PTSD appears to be associated with altered activity of the hypothalamic-pituitary-adrenal (HPA) axis (
3). However, a meta-analysis revealed that hypocortisolism is not a consistent finding in PTSD (
4). In addition, there is evidence for higher glucocorticoid sensitivity of the HPA axis in PTSD (
5,
6), but this may be induced by military deployment independently of the disorder (
7,
8). Regulation of immune responses by glucocorticoids also seems to be altered in PTSD patients. Our group previously reported lower glucocorticoid sensitivity of T-cell proliferation and higher glucocorticoid sensitivity of lipopolysaccharide-stimulated cytokine production in PTSD patients (
9). Accordingly, higher in vitro inhibition of lipopolysaccharide-stimulated production of cytokines (
10) and lysozymes (
11) by glucocorticoids has been observed in PTSD patients as well. However, at least part of the relation between PTSD and glucocorticoid regulation of the immune response could be attributed to trauma exposure, since trauma-exposed individuals without the disorder also displayed alterations in glucocorticoid sensitivity of the immune system compared with healthy subjects (
9,
11).
Glucocorticoid receptors (GRs) and mineralocorticoid receptors mediate glucocorticoid actions. Mineralocorticoid receptors are saturated at low basal cortisol levels, while GRs play a key regulatory role during periods of increased cortisol (e.g., in stressful conditions) (
12). GRs reside in the cytosol and, upon ligand binding, translocate to the nucleus to regulate gene transcription (
13). GR sensitivity is influenced by heat-shock proteins (HSP70/HSP90) chaperoning GRs, which influence proper folding and maturation of the GR and subsequent translocation and gene transcription (
14).
FKBP5, both target gene and cochaperone of the GR-HSP70/90 heterocomplex, can lower GR affinity and thereby alter glucocorticoid binding (
15).
In the present study, we investigated, before deployment, whether military personnel with and without deployment-related onset of PTSD symptoms differed in dexamethasone binding capacity of PBMCs. Since the gluocorticoid:mineralocorticoid receptor ratio in human PBMCs is approximately 10:1 (
20), we refer to the results of the binding assay as the GR number or binding. Moreover, the predictive value of the preexisting GR number for development of PTSD symptoms after deployment was investigated. Subsequently, we assessed whether group differences existed in mRNA expression for subtypes of the GR (GR-α, GR-β, and GR-P), GR target genes (glucocorticoid-induced leucine zipper [
GILZ] and serum and glucocorticoid-inducible kinase-1 [
SGK-1]), and the GR target gene and cochaperone
FKBP5.
Method
General Procedure
This study is part of a prospective cohort study on deployment-related disorders in the Dutch Armed Forces. Individuals volunteered to participate prior to a 4-month deployment to Afghanistan. Duties during deployment included combat patrols, clearing or searching buildings, participation in demining operations, and transportation across enemy territory. The combat group was exposed to typical war zone stressors, including expo sure to enemy fire, armed combat, and seriously injured and dead fellow soldiers and civilians. The study was approved by the Institutional Review Board of the University Medical Center Utrecht (Utrecht, the Netherlands). Written consent was also obtained. One to 2 months prior to deployment and approximately 1 and 6 months after deployment, participants completed questionnaires and a heparinized blood sample was drawn between 8:00 and 11:30 a.m.
Participant Selection
Participants were selected from 455 men who completed all assessments. Analysis of GR binding and associated measures was performed by individuals blind to the participants' PTSD status. Participants were assigned to the PTSD group when their score on the Self-Rating Inventory for PTSD (
21) 6 months after deployment was ≥38. This cutoff score corresponds to the mean plus two standard deviations, which coincides with the 95th percentile of scores before deployment within a population of 704 soldiers from the Dutch Armed Forces (mean: 26.91 [SD=5.34]). The validity of this cutoff score is supported by van Zelst et al. (
22), who compared Self-Rating Inventory for PTSD scores and ratings from a diagnostic interview within a community population of older adults.
Since we investigated PTSD onset, eight participants with scores ≥38 before deployment were excluded. All other participants fulfilling the selection criteria for the PTSD group were included (N=34). Subsequently, a comparison group (N=34) was identified by matching, to participants in the PTSD group, for age, period of deployment, function and rank during deployment, education, and number of previous deployments. Scores had to be below the cutoff score at all time points. Since we previously found that depressive symptoms are related to high GR binding in PBMCs, participants with high levels of depressive symptoms before or after deployment (Symptom Checklist-90 depression subscale score ≥24 [19]) were excluded from the comparison group.
Matched comparison subjects were compared with non-selected participants without high levels of PTSD or depressive symptoms before deployment or 6 months after deployment. Matched comparison subjects were somewhat younger (matched comparison subjects: mean age=26.68 years [SD=9.28]; nonselected comparison subjects: mean age=29.42 years [SD=9.06]; t=−1.679, df=380, p=0.09) and had experienced fewer previous deployments (matched comparison subjects: mean=0.65 [SD=0.85]; nonselected comparison subjects: mean=1.06 [SD=1.34]; t=−2.547, df=50.71, p=0.01) than nonselected comparison subjects. There were no group differences in year of deployment, rank and function during deployment, and education.
Measures
Questionnaires
PTSD symptom level over the past 4 weeks was assessed with the 22-item Self-Report Inventory for PTSD. A higher score indicates more PTSD symptoms (range: 22-88). This inventory has good concurrent validity with other PTSD measures, such as the Clinician Administered PTSD Scale and the Mississippi Scale for PTSD (
21). Level of depressive symptoms was assessed using the Dutch version of the 16-item Symptom Checklist-90 depression subscale (
23). A higher score on this measure indicates more depressive symptoms (range: 16-80). Exposure to potentially traumatic experiences before the age of 18 was assessed using the Dutch version of the short-form Early Trauma Inventory-Self-Report (
24). The questionnaire consists of 27 dichotomous items. The total score represents the number of experienced events. Exposure to potentially traumatic deployment stressors was assessed with a 13-item checklist specifically developed for the present study (see the data supplement accompanying the online version of this article).
Dexamethasone Binding
For determination of the capacity of PBMCs to bind glucocorticoids, a validated whole cell single-point binding assay was used, as described previously (
19). This method provides a reliable estimate of B
max, as determined using a classical binding assay with 3-200 nM
3H-dexamethasone (r
2=0.92) (
19). Briefiy, PBMCs were isolated from whole blood using Ficoll-Paque (Pharmacia and Upjohn, Uppsala, Sweden), and 10
7 cells were frozen in dime-thyl sulfoxide. After thawing and 60 minutes of equilibration in a culture medium, cells were washed twice, resuspended in assay buffer (RPMI-1640, with 5% fluorescence correlation spectroscopy), and incubated in duplicate with 100 nM
3H-dexamethasone (Amersham, Buckinghamshire, United Kingdom) in the presence or absence of excess unlabeled dexamethasone (Sigma-Aldrich, Steinheim, Germany) for 1 hour at 37°C. Cell-bound radioactivity was quantified by liquid scintillation analysis.
mRNA Expression
Total RNA was isolated from PBMCs with Trizol (Invitrogen, Carlsbad, Calif.). One μg of total RNA was used to synthesize cDNA with SuperScript Reverse Transcriptase (Invitrogen, Carlsbad, Calif.). Real-time polymerase chain reactions were performed with an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, Calif.) (see
Table 1 for primer sequences). Data were normalized for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin expression.
Cortisol
Plasma cortisol levels were measured by electrochemiluminescence immunoassay on the Modular E170 (Roche Diagnostics, Mannheim, Germany). Lower limit of detection was 3 nmol/l; interassay variation was <3%; and reference values (7:00-10:00 a.m.) were 170-540 nmol/l.
Leukocyte Subset Analysis
Leukocyte subsets were assessed by dual-color fluorescence analysis with a Becton Dickinson Calibur flow cytometer (Becton, Dickinson and Company, Franklin Lakes, N.J.) to quantify cluster of differentiation (CD) 14+ (monocytes), CD3+ (T cells), CD4+ (T helper/inducer), CD8+ (T suppressor/cytotoxic), and CD19+ (B cells), as described previously (
19).
Data Analysis
Analyses were performed using SPSS, Version 15.0 (SPSS, Inc., Chicago). A p value <0.05 (two-tailed) was considered significant. Variables were tested for normality and log-transformed when necessary. Outliers were removed if z values fell outside the range of ±3.29 (GR-α: comparison group, N=1; GR-β: PTSD group, N=1; GR-P: comparison group, N=1; FKBP5: PTSD group, N=2; GILZ: PTSD group, N=1; SGK-1: PTSD group, N=2). Group differences were tested with t tests and analysis of covariance for continuous parametric variables and with chi-square tests for categorical variables. Mann-Whitney tests were used for nonparametric log-transformed variables. Logistic regression analysis with group as a dependent variable, controlling for potentially traumatic childhood experiences and predeployment questionnaire scores for PTSD and depressive symptoms, was performed to test the predictive value of the preexisting GR number for PTSD status after deployment. This analysis provided the odds ratio for inclusion in the PTSD group with each GR increase of 1,000. Linear regression analysis was performed with the preexisting GR number as a dependent variable to test whether the GR number was associated with predeployment participant characteristics. For regression analyses missing variables for predeployment Symptom Checklist-90 depression sub -scale scores (PTSD group: N=1), the number of previous deployments (PTSD group: N=1), and body mass index (PTSD group: N=3) were imputed in SPSS using the option “linear trend at point.”
Discussion
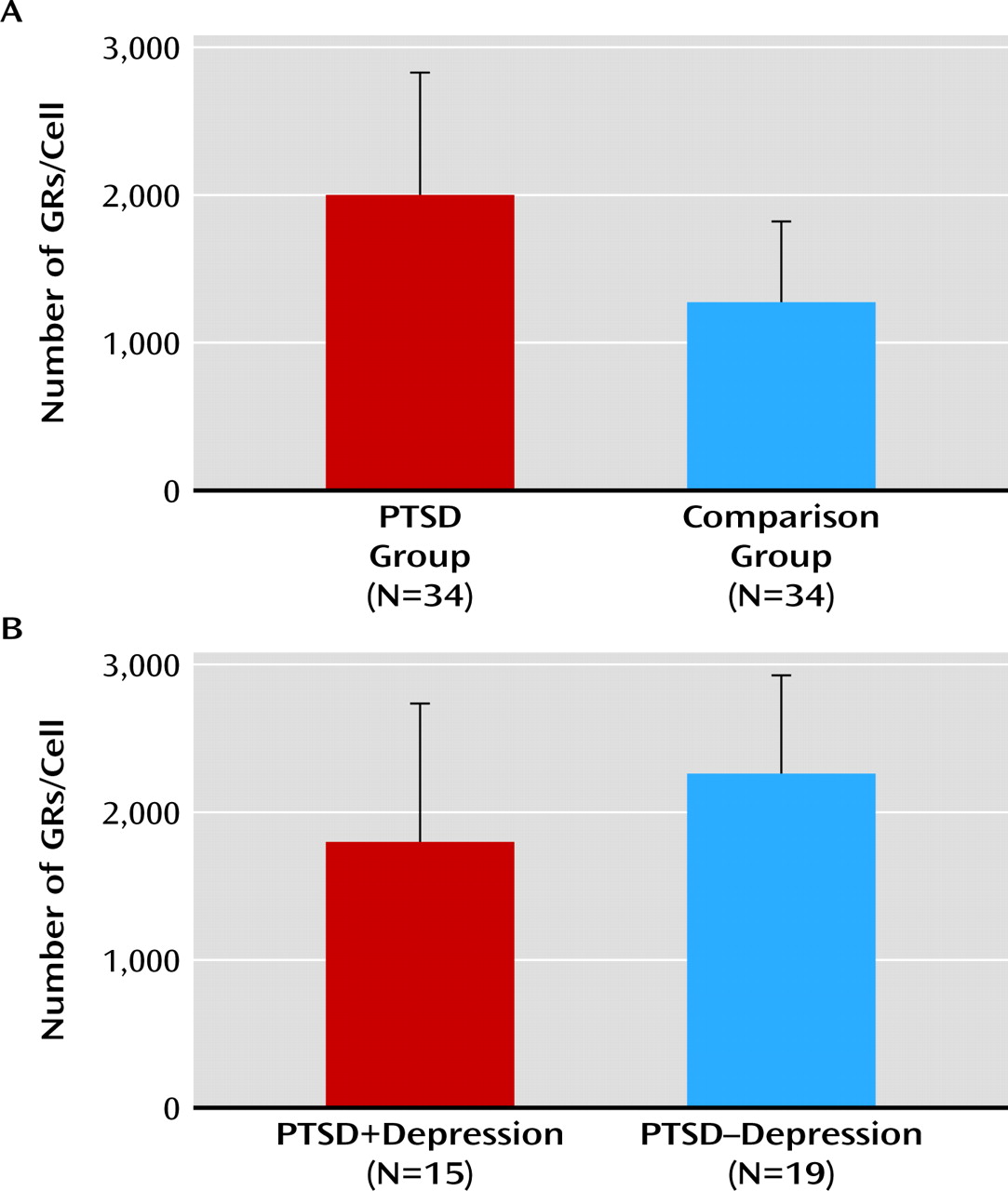
This study reveals that high preexisting dexamethasone binding capacity of PBMCs, reflecting a high GR number in these cells, represents a vulnerability factor for development of PTSD symptoms after military deployment. Before deployment, a higher GR number in PBMCs was present in military personnel who developed a high level of PTSD symptoms 6 months after deployment. The predeployment GR number was a strong predictor of inclusion in the PTSD group after deployment; with each increase of 1,000 GRs, the odds ratio for the presence of PTSD symptoms after deployment increased 7.5-fold. The observed group difference was still present after deployment.
Although the PTSD group reported more predeployment symptoms of PTSD and depression, the predeployment GR number was not influenced by these predeployment symptoms. Furthermore, group differences in the PBMC composition also did not influence the results. In addition, group differences in the GR occupancy by endogenous glucocorticoids most likely did not influence the observed group differences, since plasma cortisol levels did not differ between groups. Moreover, cells were equilibrated in a culture medium and extensively washed before the addition of 3H-dexamethasone to prevent possible effects of binding of endogenous glucocorticoids.
We previously reported a higher preexisting GR number in PBMCs among military personnel with depressive symptoms and severe fatigue after deployment (
19). In the present study, almost one-half of the participants in the PTSD group reported high levels of depressive symptoms after deployment, which is consistent with comorbidity estimates for PTSD and major depressive disorder in war veterans (
25). However, there was no difference in the pre-deployment GR number between participants with and without depressive symptoms in the PTSD group. Therefore, the present finding is related to development of PTSD symptoms after deployment and not to development of depressive symptoms in a subgroup of participants.
Although changes in GR functioning in PTSD and major depressive disorder are supposed to be different (
3,
26), a high preexisting GR number in PBMCs is related to both PTSD and depressive symptoms after deployment (
19). Thus, a high preexisting GR number in PBMCs may be a predictor of mental health problems specifically induced by stress and/or trauma.
A limitation of the present study is that mental health status was not assessed with structured clinical interviews. Therefore, it is uncertain whether participants in the PTSD group fulfilled DSM-IV diagnostic criteria for PTSD. Individuals with a subclinical level of PTSD symptoms, therefore, might also have been included in the PTSD group. However, it is reasonable to assume that the questionnaires are a reliable reflection of the presence of PTSD symptoms (
21).
Currently, various subtypes of the GR have been identified. GR-α is the most abundant variant and is transcriptionally active (
27), while GR-β is less ubiquitously expressed, does not bind glucocorticoids, and has limited transcriptional capability (
28). Additionally, GR-P is widely expressed and increases GR-α activity in cell lines (
29,
30). To our knowledge, there are no published data on GR mRNA expression levels in PTSD. However, within major depressive disorder, the peripheral GR number (for review see reference
26) and peripheral GR-α mRNA expression (
31) both appear to be decreased. Our results show that the observed group difference in the GR number in PBMCs was not accompanied by group differences in GR mRNA expression level. This result implies that the higher GR number in the PTSD group is mediated by posttranscriptional mechanisms. Additionally, we cannot completely exclude that a group difference in mineralocorticoid receptor level influenced the observed higher dexamethasone binding capacity of PBMCs in the PTSD group.
Alterations in
FKBP5 expression influence glucocorticoid binding capacity of PBMCs (
15). Segman et al. (
32) reported that the amount of up-regulation of
FKBP5 mRNA expression after trauma predicted the presence of PTSD 4 months later. In addition, Yehuda et al. (
33) found decreased
FKBP5 mRNA expression levels in PTSD patients. In our study, however, there was no group difference in
FKBP5 mRNA expression before deployment. We also did not observe group differences in the GR target genes
GILZ and
SGK-1. It may well be possible, however, that compensation by other pathways that regulate these genes compensate for a putative difference in GR activity.
The observed higher GR binding in the PTSD group may be associated with the presence of specific GR polymorphisms, which could, for example, alter posttranscriptional mechanisms influencing GR protein expression or directly affect the binding capacity of the GR. Bachmann et al. (
34) reported no association between PTSD and the presence of GR polymorphisms BC1I and N363S. Currently, however, more polymorphisms in the GR gene have been identified (for review see reference
35), which were not yet investigated in relation to development of PTSD at the time of the study. Whether genetic factors are involved in the higher GR number in PBMCs observed in the PTSD group will be investigated in a larger sample.
In our study, participants in the PTSD group experienced almost twice as many potentially traumatic events during childhood than comparison subjects. Although the number of traumatic experiences during childhood was not related to the GR number in PBMCs, it is possible that a higher GR number in PBMCs and exposure to childhood trauma together increase the vulnerability for PTSD development after deployment. In this context, Bet et al. (
36) reported that the co-occurence of both the GR polymorphisms 22/23EK and 9β with childhood trauma is associated with increased risk for development of major depressive disorder.
Peripheral GRs are often considered to be an accessible model for GR expression in the brain. Nonetheless, whether the mechanisms of regulation of central and peripheral GRs are similar has not been studied extensively. In rats, neuronal and lymphoid cytosolic GRs are similar in glucocorticoid affinity and specificity (
37), and cytosolic GRs in the brain and peripheral immune tissues are down-regulated after chronic corticosterone administration following adrenalectomy (
38,
39). It remains unknown whether and how the observed higher predeployment GR number in PBMCs is involved in the pathophysiology of PTSD. We speculate that our finding of a higher GR number in PBMCs may be paralleled in the brain. If a suitable radiotracer could be found, positron emission tomography neuroreceptor mapping of GRs could provide valuable information about GR-mediated abnormalities in the brain (
40).
The present study is the first, to our knowledge, to show that a high GR number in PBMCs before military deployment was a strong predictor of the presence of PTSD symptoms after deployment and was maintained until at least 6 months after deployment. We propose that a high GR number in PBMCs may be a biomarker of increased risk for development of PTSD symptoms after trauma exposure.