Despite the paucity of supporting data, antipsychotic polypharmacy remains a prevalent practice (
1,
2), with estimates of antipsychotic polypharmacy among individuals with schizophrenia typically ranging from 10% to 30% (
3,
4). Moreover, the rate of polypharmacy appears to be increasing over time (
3,
5); a study of Medicaid claims for more than 30,000 Medicaid recipients with schizophrenia reported an increase from 32% in 1998 to 41% in 2000 (
3). In contrast, guidelines for the treatment of schizophrenia either are silent on antipsychotic polypharmacy (
6,
7) or recommend it only as a last resort (
8).
To date, the only randomized controlled trials examining antipsychotic polypharmacy focused on combinations with clozapine (
9–
13). Simpler prescribing regimens are commonly assumed to be associated with improved adherence, fewer side effects, and lower costs (
1,
8,
14). On the other hand, switching from polypharmacy to monotherapy may present clinical challenges. In one small open-label study in which 44 patients switched from polypharmacy to monotherapy, symptoms in 10 patients (23%) worsened significantly, while those in 24 patients (55%) remained stable and those in 10 patients (23%) improved (
15).
Given concerns about the risks (
14) and costs (
5) of antipsychotic polypharmacy, some large public mental health systems have begun initiatives to reduce its prevalence. For example, the New York State Office of Mental Health convened a scientific advisory board to identify clinically questionable prescribing practices, and polypharmacy was the one area identified as raising clinical concerns by each of the groups of experts convened (
1). Subsequently, the authors of a meta-analysis based mainly on studies involving polypharmacy with clozapine (
16) concluded that in certain clinical situations, antipsychotic polypharmacy may be superior to monotherapy, but they also noted that the availability of studies may be subject to publication bias.
Antipsychotic polypharmacy is thus a common practice that persists despite a lack of supporting evidence and despite treatment guidelines that discourage it. Polypharmacy raises concerns about increases in total dosages, side effects, and mortality, as well as decreased adherence (
14). The limited number of studies comparing antipsychotic polypharmacy and monotherapy suggests the need for randomized trials directly addressing this issue. We report here on a trial examining whether a patient with schizophrenia on polypharmacy with two antipsychotics is likely to be better off remaining on both antipsychotics or discontinuing one of them.
Method
Study Participants
Between December 2004 and March 2008, 15 study sites in the National Institute of Mental Health's Schizophrenia Trials Network and five sites in Connecticut's public mental health system recruited patients 18 years of age and older who had a diagnosis of schizophrenia or schizoaffective disorder (according to the Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition [SCID-P];
17) and were currently taking two prescribed antipsychotic medications, documented by a plasma level greater than zero for each antipsychotic. Additional inclusion criteria were persistent psychopathology or significant side effects; willingness to consider a change in antipsychotic medication; continuing access to medications without financial burden; and at least one clinical visit every 3 months for the past 6 months. Exclusion criteria included having symptoms or side effects so severe that a medication change was indicated immediately; having an exacerbation of psychiatric symptoms within the past 3 months resulting in significant intervention, such as having spent one or more nights in psychiatric hospitalization or having received services from a crisis intervention program or psychiatric emergency department; living in a skilled nursing facility as a result of a physical condition or disability; having pending criminal charges; currently pregnant or breastfeeding; and currently receiving three or more antipsychotic medications for ongoing daily administration. For patients with prescriptions for quetiapine, the dosage had to be at least 100 mg/day (in order to exclude those for whom this agent was prescribed primarily as a sleep aid).
The study was carried out with the approval of the participating institutions' institutional review boards. After potential participants received a thorough description of the study, an assessment of understanding of the consent material was conducted and clinical interviewers obtained each individual's written informed consent (see the data supplement that accompanies the online edition of this article for a CONSORT diagram detailing the recruitment flow). Next, patients completed a baseline interview that included collection of a blood sample to determine adherence to prescribed antipsychotics.
After completion of the baseline interview and receipt of laboratory findings confirming the presence of both prescribed antipsychotics, the study's project director used a single predetermined randomization stream (i.e., without stratification) to assign participants either to stay on both antipsychotics or to discontinue one (switch to monotherapy). No exceptions were made to this predetermined randomization stream. For participants who were assigned to switch to monotherapy, each participant and physician decided together which of the two antipsychotics to discontinue. The study protocol indicated that discontinuation had to occur within 30 days, and participants had to continue with their assigned medication regimen for 6 months unless this treatment was clinically contraindicated. Medication dosing was not constrained by the study protocol; instead, prescribers used their clinical judgment to adjust dosages as they believed best for the patient within the assigned treatment condition. The study protocol did not restrict the use of adjunctive or concomitant psychotropic medications other than antipsychotic medications. After the 6-month study period, data collection continued for an additional 6 months of naturalistic follow-up. Treatment was open label with assessment by blinded clinical raters.
Baseline Measures
Clinician interviewers used the SCID-P to obtain a research diagnosis. Additionally, interviewers recorded sociodemographic information and psychiatric history, drawn from both chart review and participant interview.
Primary outcome measure.
The primary outcome measure was time to all-cause medication discontinuation. Project staff at each site conducted record reviews and provided start and stop dates for each dosage of each medication prescribed. The project director reviewed each form and followed up with sites as needed to clarify any discrepancies and to verify accuracy.
Secondary outcome measures.
Secondary outcome measures included measures of psychiatric symptoms, hospitalization, and medication side effects. Interviewers conducted assessments with study participants at baseline and at six follow-up points: 2 weeks, 1 month, 3 months, 6 months, 9 months, and 12 months after condition entry, defined as the date participants were informed of and began their randomized treatment assignment.
We used the Positive and Negative Syndrome Scale (PANSS;
18) to assess psychiatric symptoms. We obtained the dates of inpatient hospitalizations using a self-report calendar augmented by record review; we included information about hospitalizations 6 months prior to baseline through study end.
To assess extrapyramidal side effects, we used the Abnormal Involuntary Movement Scale (AIMS;
19) and the Simpson-Angus Rating Scale (
20). We assessed sexual dysfunction using the Arizona Sexual Experience Scale (
21). We measured the perceived awareness of and distress from common side effects of antipsychotic medications using the Subjective Side Effect Rating Scale (
22), and we asked participants additional questions regarding weight change (e.g., whether they had to get new clothes because their old clothes did not fit anymore, whether they were actively trying to gain or lose weight). We recorded each participant's pulse, blood pressure, height, weight, waist and hip measurements, serum prolactin levels, lipid panel results, and blood and urine glucose levels. For women, interviewers also used the urine samples to test for pregnancy at the 6- and 9-month interviews. Other data collected will be the subject of future reports.
We adapted Schooler and Kane's (
23) research criteria for tardive dyskinesia (at least “moderate” movements in one or more body areas or at least “mild” movements in two or more body areas as rated on the AIMS) to identify instances of onset of tardive dyskinesia following condition entry. We defined possible new-onset extrapyramidal symptoms as an average increase of >0.3 across items on the Simpson-Angus Rating Scale.
Rater training and reliability.
Clinicians with at least a master's degree who had clinical experience with people with schizophrenia conducted all interviews. To maintain blinding, randomization was managed centrally. When the project director alerted participating sites of the random assignment, she reminded them not to discuss the randomization with the study raters. All electronic files containing information about the randomized condition were protected with a password known only to research staff members who were not blinded to the study condition, and any paper documentation that included treatment assignment (e.g., the list of medications the phlebotomist used to label blood collection vials) was clearly marked “Confidential, please do not share with raters.” After randomization, all medical record reviews and blood shipments were conducted by research staff who were not blind to the study condition. In addition to these procedures, we asked raters to begin all telephone conversations with study participants by reminding them not to mention the medications they were taking, how many medications they were taking, whether they changed medications, or whether they received a shot or took a pill (or both). At the beginning of each follow-up interview and before sections where unblinding was most likely to occur, the rater read scripted language reminding the participant that the rater could not know the medication(s) that the participant was taking (as above). In rare cases where raters became aware of the medication the participant was taking, the interview was stopped and resumed with a different rater who was blind to the study condition.
Before being certified to conduct assessments, raters participated in an initial training on the SCID, PANSS, and AIMS conducted by staff of the Schizophrenia Trials Network. Raters also completed an annual retraining to maintain certification. Both the initial training and the annual refresher training involved rating a combination of clinical vignettes (for the SCID) and video recordings (for the PANSS and AIMS) and achieving threshold scores for each instrument.
Data Analysis
Paralleling the analytic approach of phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE;
24) and subsequent analyses of the impact of switching antipsychotic medications using CATIE data (
25), we used Kaplan-Meier and Cox regression analysis to examine the impact of staying on polypharmacy compared to switching to monotherapy on time to all-cause treatment discontinuation, with gender and race (Caucasian versus non-Caucasian) as covariates because the groups differed significantly on these measures at baseline. Similarly, we applied mixed models to examine the effect of staying or switching on study measures throughout the 6-month study period (i.e., during the period of protocol-driven treatment and excluding the naturalistic follow-up); independent variables included group (stay or switch), time (linear and quadratic), and group by linear and quadratic time with gender and race as covariates. We used intent-to-treat models for our primary analyses in which a significant group-by-time interaction would support the hypothesized difference. Secondarily, we also examined two as-treated models, one that excluded participants who had discontinued their assigned treatment condition entirely and one in which data from such individuals were excluded only after discontinuation of the assigned treatment. For participants assigned to the stay condition, discontinuation of the assigned treatment was defined as discontinuing either of the antipsychotics taken at baseline (adding a third antipsychotic also would have been deemed a discontinuation of the baseline treatment, but this did not occur). For participants assigned to the switch condition, discontinuation of the assigned treatment was defined as switching to a second monotherapy agent after first discontinuing one of the two antipsychotics taken at baseline or adding an additional antipsychotic (i.e., returning to polypharmacy).
Results
Table 1 lists the baseline values for the stay and switch groups on various measures. On average, the groups were receiving comparable dosages of antipsychotics at study entry; baseline dosages were higher among those switched to monotherapy, but this difference fell short of statistical significance. Dosages were within the ranges recommended by the Schizophrenia Patient Outcomes Research Team guidelines (
6). The most common baseline polypharmacy combinations were quetiapine and risperidone (N=25), quetiapine and a first-generation antipsychotic (N=25), risperidone and a first-generation antipsychotic (N=23), olanzapine and a first-generation antipsychotic (N=22), ziprasidone and a first-generation antipsychotic (N=12), aripiprazole and quetiapine (N=11), olanzapine and risperidone (N=10), and other combinations totaling 10 or fewer individuals each (N=39). While the study's inclusion criteria required the presence of either residual symptoms or side effects, every participant who entered the trial experienced residual symptoms, irrespective of whether they also experienced any side effects.
Of the 127 patients included in the randomization process, 114 began their assigned treatment (stay on polypharmacy, N=56; switch to monotherapy, N=58). Of these 114, eight (14%) of those assigned to stay on polypharmacy discontinued their assigned treatment (six changed to a different polypharmacy combination, and two discontinued one of the two assigned medications). Reasons for discontinuation included increased symptom levels (N=2), participant preference (N=1), and side effects (N=5; two with weight gain, one with extrapyramidal side effects, one with diabetes, and one with unspecified side effects). Among those assigned to switch to monotherapy, 18 (31%) discontinued their assigned treatment (12 returned to their baseline polypharmacy combination, three began a different polypharmacy combination, one began monotherapy with an agent other than one of the two baseline medications, one began monotherapy with one of the baseline agents and changed to monotherapy with the second baseline agent after 90 days, and one began to taper one of the two agents but did not complete the taper after symptom levels increased). Reasons for discontinuation included increased symptom levels (N=11), participant preference (N=6), and side effects (N=1 with hyperlipidemia).
Of the 58 participants assigned to switch to monotherapy who began the treatment, 12 (21%) discontinued quetiapine, 10 (17%) discontinued risperidone, nine (15%) discontinued olanzapine, eight (14%) discontinued haloperidol, and the remaining 19 (33%) discontinued other antipsychotics (each at less than 10% of discontinuations). Among the 30 polypharmacy combinations that consisted of a first- and a second-generation antipsychotic, the second-generation antipsychotic was continued in slightly over half of the cases (18 of 33 such combinations; 5/10 for quetiapine and a first-generation antipsychotic; 5/9 for olanzapine and a first-generation antipsychotic; 3/7 for risperidone and a first-generation antipsychotic; 2/4 for ziprasidone and a first-generation antipsychotic; and 3/3 for aripiprazole and a first-generation antipsychotic).
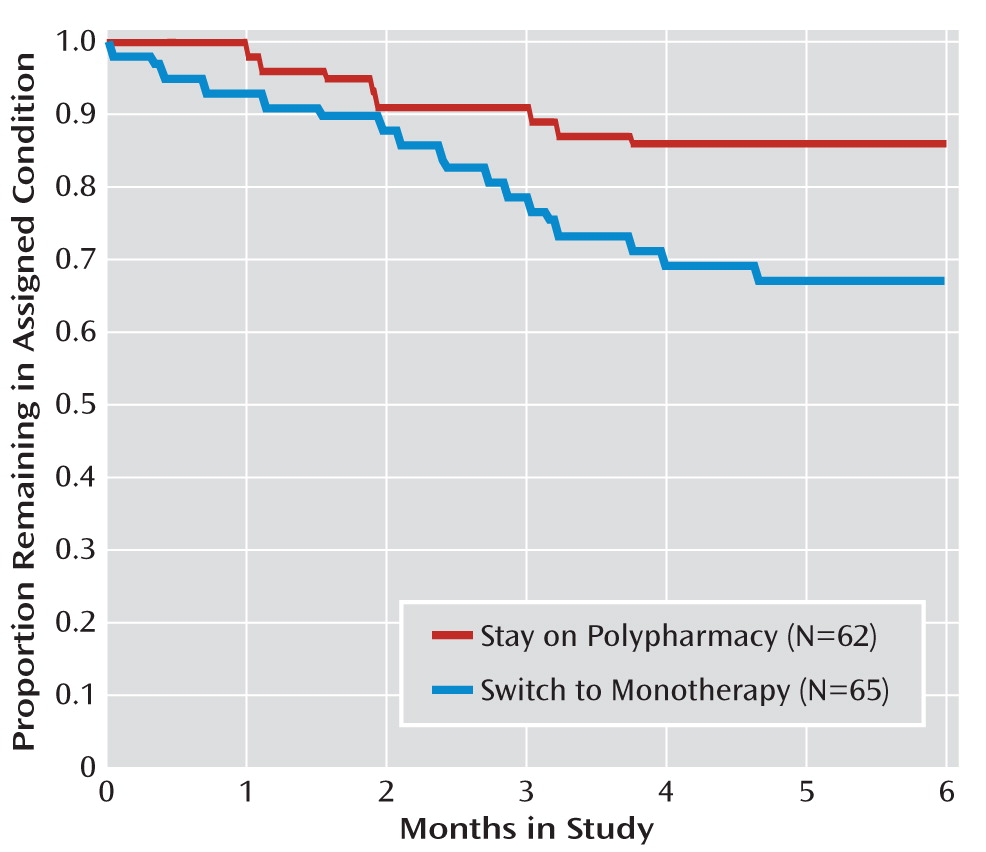
Time to all-cause treatment discontinuation (defined as time to change in antipsychotic medication for any reason) was shorter for participants assigned to switch from polypharmacy to monotherapy than for those assigned to stay on polypharmacy, and switching from polypharmacy to monotherapy resulted in treatment discontinuation significantly more often than did continuation of polypharmacy (
Figure 1). This difference remained significant above and beyond gender and race in Cox regression analyses (
Figure 1).
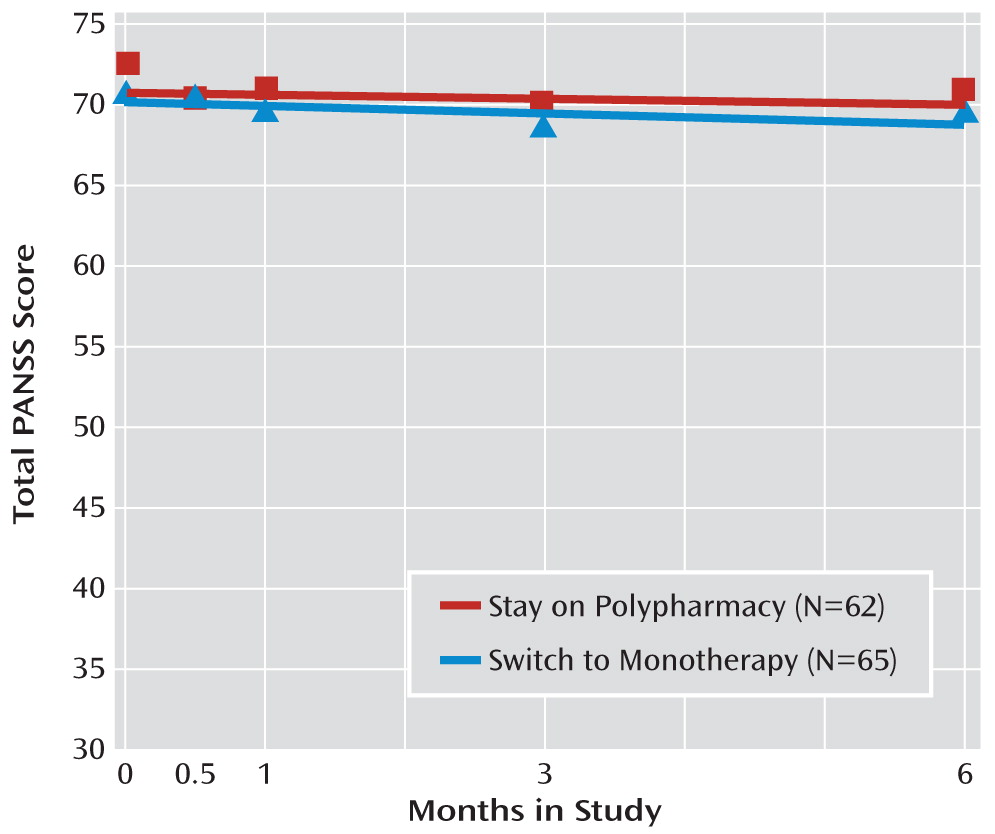
The stay and switch groups did not differ significantly on psychopathology over time in random regression models (
Table 2;
Figure 2), whether measured as total PANSS score, total PANSS positive score, or the five factors defined by Marder and colleagues (
29). The sole exception to this was a statistically significant (p=0.03) difference for the factor “uncontrolled hostility” (each group had scores very near “no symptoms” on this scale, and the groups differed by less than 0.4 points on the 24-point scale—a small effect size (0.17) and unlikely to be clinically significant—with the change over time favoring the stay condition).
The two groups also did not differ with respect to incidence of sexual side effects (
Table 2), new-onset extrapyramidal symptoms (12 patients in each group; 30% of those assigned to stay and 29% of those assigned to switch), or new-onset tardive dyskinesia (eight patients and nine patients in the stay and switch groups, respectively [19% for both groups]). The groups did not differ with respect to the likelihood of being hospitalized for psychiatric reasons, which was uncommon in each group (five [8%] and seven [11%] hospitalized at least once during the first 6 months for the stay and switch groups, respectively), nor did the groups differ with respect to time to first hospitalization for psychiatric reasons. The groups also did not differ with respect to total hospitalizations (medical and psychiatric).
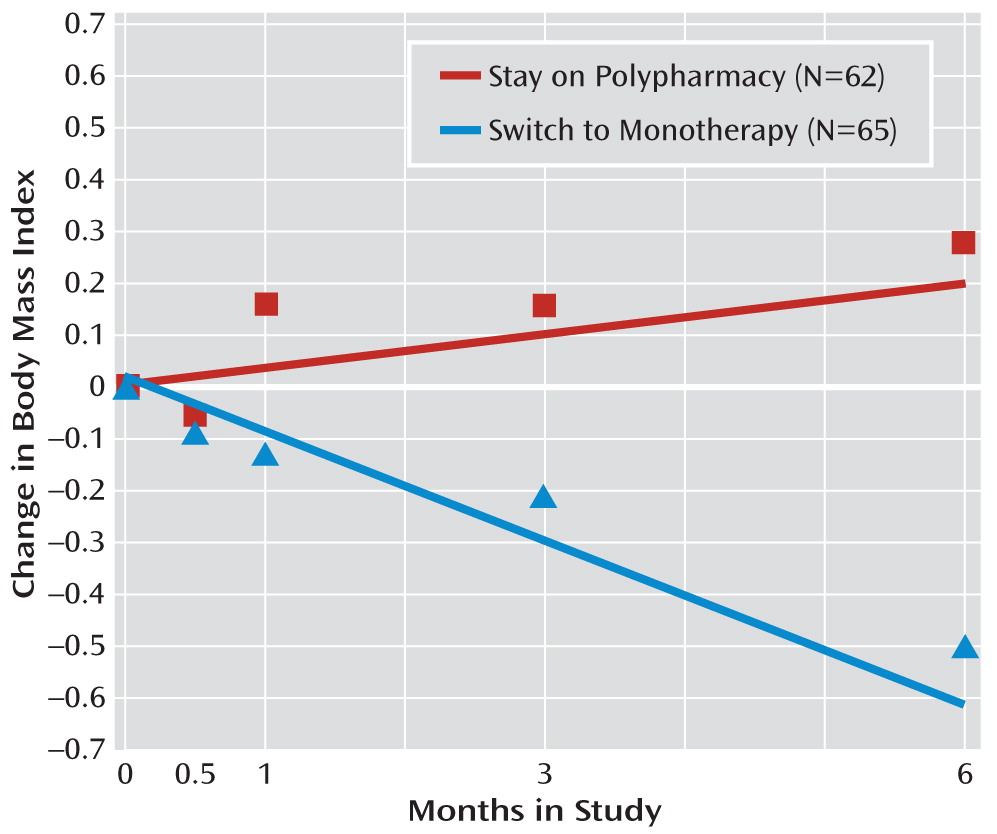
Body mass index (BMI) decreased significantly among patients assigned to switch to monotherapy compared to those assigned to stay on polypharmacy, in whom BMI increased (
Table 2;
Figure 3; group-by-time interaction, p=0.05). Over the 6 months of the study, on average, among participants in the switch group, BMI decreased by 0.50 points (SD=2.32), and among those in the stay group, it increased by 0.28 points (SD=2.31). Results of each of the secondary (as-treated) models were consistent with those of the primary intent-to-treat analyses.
Discussion
This is the first report of a randomized trial examining the effectiveness of switching patients with schizophrenia from antipsychotic polypharmacy to monotherapy. In terms of time to all-cause discontinuation, remaining on polypharmacy was superior to switching to monotherapy, reflecting a greater likelihood of dissatisfaction on the part of the patient or physician with the switch to antipsychotic monotherapy than with staying on polypharmacy. Patients who discontinued one antipsychotic were more likely to change their treatment—and on average did so sooner—than those who stayed on polypharmacy, with the overwhelming majority of these individuals simply going back to their previous polypharmacy regimen.
These findings are consistent with results of a nonrandomized open-label study of discontinuing polypharmacy in which 44 individuals were tapered from polypharmacy to monotherapy (
15); over half (54%) of the patients remained stable, 23% showed improvement, and 23% fared more poorly.
Because this was an open-label trial with blinded raters, for patients in the stay condition, patients and prescribers both knew that any changes in symptoms or side effects that were temporally associated with beginning the trial were not due to medication changes. In contrast, for patients in the switch condition, since any such differences were temporally related to a medication change, some may have believed, correctly or not, that this temporal relationship indicated causality. Thus, it may be that the patients and prescribers in the switch condition were more inclined to attribute alterations in feelings, symptoms, or side effects to the change in medication than were those in the stay condition, who may have experienced these same alterations as part of normal variations in illness and medication response. This attribution may have prompted patients in the switch condition to change medications sooner than they would have if they had experienced the same changes in symptoms or side effects while continuing on a familiar polypharmacy regimen. Hence, time to all-cause discontinuation may be a more appropriate measure for double-blind trials in which prescriber and patient expectations are controlled and both study conditions include newly started medications. Rater blinding helped protect ratings from these potential biases, and the single difference seen (PANSS hostility scores favoring the stay condition), while statistically significant, is unlikely to be clinically significant.
Our results support the reasonableness of policy makers and others constructing prescribing guidelines that encourage trials of antipsychotic monotherapy for patients receiving antipsychotic polypharmacy, with the caveat that patients should be free to return to the polypharmacy combination if an adequate trial of antipsychotic monotherapy proves unsatisfactory (
1). The study demonstrated that such switches to monotherapy can be accomplished successfully for the majority of patients—69% of those who switched to monotherapy remained on monotherapy for the 6-month study period. On average, switch to monotherapy resulted in loss of body mass, with no worsening of symptom control and no increase in hospitalization. At 6 months, among patients assigned to switch to monotherapy, BMI had decreased by a mean of 0.5 points, compared with an average gain of 0.3 points for those who continued on polypharmacy. This net difference of 0.8 points corresponds to a net of 5 pounds for an individual who is 5 feet 7 inches tall and weighs 203 pounds—the mean baseline height and weight of our study participants. Treatment guidelines recommend considering a change in antipsychotic medication when an individual gains one BMI point (
30). In the analyses, we controlled for the significant baseline group differences in gender and race to allow assessment of staying on polypharmacy compared with switching to monotherapy independent of race and gender. Hence we did not explore whether race or gender moderated treatment outcomes. Similarly, although not significant, the slightly higher baseline dosages among patients assigned to monotherapy may have influenced the results. Study participants were drawn from the broad pool of patients on antipsychotic polypharmacy for whom a medication change is a clinically viable option.
As data from the CATIE study illustrated, it is difficult to improve on staying on the currently prescribed single antipsychotic (
25). CATIE data were useful in comparing the relative effectiveness of staying on a given antipsychotic monotherapy to switching to a different monotherapy. The results of the present study indicate that most patients with continuing psychopathology while taking two antipsychotic medications are well able to tolerate switching from polypharmacy to monotherapy, and they reap the metabolic benefits associated with weight loss while avoiding the side effects and other burdens of taking an additional medication.
While this study yields no information on whether starting polypharmacy is a good idea in the first place, it does make it clear that patients on two antipsychotics may benefit from discontinuing one of the medications.
Acknowledgments
The authors thank Linda Frisman, Robert Gibbons, Philip Harvey, John Kane, and Peter Weiden for their suggestions during the design and implementation phases of this study; Jennifer Manuel and Sue Marcus for help with the analyses; and Patrick Corrigan, Psy.D., Lisa Dixon, M.D., Andrew Leon, Ph.D., Steve Marder, M.D. (chair), Delbert Robinson, M.D., Yvette Sangster, and Larry Siever, M.D., for serving on the study's data safety monitoring board.