Several medications have been approved by the U.S. Food and Drug Administration for the treatment of alcohol dependence—disulfiram, naltrexone (both oral and long-acting injectable), and acamprosate—and a few other medications, such as topiramate, have been shown to be effective in treating alcohol dependence. However, many individuals respond only partially or not at all to these agents. There is a need for new medication treatments, especially ones using agents that target different aspects of alcohol dependence, ranging from individual phenomenological to genetic differences. One such phenomenological difference may be defined by a set of signs and symptoms that might change over time as individuals attempt to stop drinking. For instance, it is well known that the period immediately after cessation of alcohol consumption is a high-risk time for relapse drinking. This early abstinence period might be defined by certain phenomena that are likely to ameliorate over time with continued abstinence. These signs and symptoms could range from easily observed alcohol withdrawal symptoms to a more subtle but still meaningful constellation of problems, including sleep difficulty, irritability, concentration problems, anxiety, and dysphoria. Some have labeled this constellation of lingering symptoms that occur after the initial cessation period as “protracted alcohol withdrawal” (
1). While this symptom constellation is not well defined, it is likely that some of these symptoms are experienced by those who do not show florid alcohol withdrawal symptoms on cessation of drinking. Nevertheless, this constellation of symptoms, and the associated craving that could manifest secondary to them, might not be particularly responsive to medications that reduce alcohol reinforcement or cue-induced craving, such as naltrexone (
2–
4). Since clinicians often cannot predict, a priori, what sort of symptoms an individual will exhibit or what sort of craving (withdrawal based versus reward based) will be the most salient, it might be wise to attempt to reduce both in order to improve treatment efficacy.
We and others have shown that anticonvulsants in general (
5–
7) and gabapentin in particular (
1,
8,
9) can ameliorate acute alcohol withdrawal symptoms and could actually be useful in preventing relapse (
10), especially in patients with previous alcohol withdrawal symptoms (
11). Gabapentin, which works by modulating both γ-aminobutyric acid (GABA) and glutamate tone, is an especially appealing drug since it hypothetically can “normalize” the known GABA deficits and glutamate excess that are thought to underlie alcohol withdrawal (
12) and perhaps parts of the early abstinence or protracted withdrawal syndrome (
13). It is also safe to use in alcoholics, as it appears to have limited or no adverse interaction with alcohol (
9,
14) and is excreted by the kidneys rather than the liver, which is known to be compromised in many alcoholics.
Method
This was a randomized controlled clinical trial approved by the institutional review board of the Medical University of South Carolina. Participants provided written informed consent after receiving a complete description of the study. After initial screening and assessment, alcohol-dependent patients were randomly assigned to receive naltrexone plus active gabapentin (N=50), naltrexone plus placebo gabapentin (N=50), or placebo naltrexone and placebo gabapentin (N=50), using a double dummy placebo-controlled medication design. Naltrexone or its matching placebo was given as 25 mg for 2 days and then 50 mg/day for up to 16 weeks. Gabapentin (300-mg capsules) or its matching placebo was given as one capsule at night (300 mg/day) on day 1; one capsule in the morning and at night (600 mg) on day 2; one capsule morning, noon, and night (900 mg/day) on days 3 and 4; and one capsule in the morning, one at noon, and two at night (1,200 mg/day) on days 5 through 42 (6 weeks).
All participants met DSM-IV criteria for alcohol dependence, consumed on average five or more standard drinks per day for men or four or more per day for women, were able to maintain sobriety for 4 days prior to randomization, and lived within 50 miles of our study site in a stable living situation. Patients were excluded if they met DSM-IV criteria for other substance dependence (except nicotine); used illicit drugs in the past 30 days or had a positive urine drug screen; met DSM-IV criteria for an axis I disorder; had current suicidal or homicidal ideation; needed maintenance with psychotropic or anticonvulsant medication; had unstable medical conditions; had liver enzyme (ALT and AST) levels more than three times normal; used disulfiram, acamprosate, or either of the study medications within the past 30 days; took an opioid medication on a routine basis; had legal charges pending; and had undergone more than one previous inpatient medical detoxification treatment.
Participants had to abstain from alcohol for at least 4 consecutive days before randomization to study medication. During the assessment period, the following instruments were administered: the Structured Clinical Interview for DSM-IV, the Alcohol Dependence Scale (
16), the Obsessive Compulsive Drinking Scale (
17), the Form 90 (a modified timeline follow-back method for documenting alcohol consumption) (
18), the Profile of Mood States (
19), the Beck Depression Inventory (
20), the Epworth Sleepiness Scale (
21), the Insomnia Severity Index (
22), and the Clinical Institute Withdrawal Assessment for Alcohol Scale–Revised (
23). Lab tests included a health screen, liver function tests, pregnancy test for females, and alcohol use markers γ-glutamyltransferase and carbohydrate-deficient transferrin (CDT) (
24).
Medication was dispensed in identically packaged blister cards. Each capsule of naltrexone, gabapentin, or placebo also contained 100 mg of riboflavin. Participants were provided up to 16 sessions of combined behavioral intervention therapy using the Combining Medications and Behavioral Interventions (COMBINE) study's combined behavioral intervention treatment manual (
25), which combined cognitive-behavioral therapy, motivation interviewing, and 12-step facilitation techniques in a client needs-based approach. A physician or nurse evaluated physical complaints and encouraged medication adherence.
Procedures
Participants were seen by a health care provider and a therapist providing combined behavioral intervention at weeks 1, 2, 3, 4, 6, 8, 10, 12, and 16 of treatment. Similar to procedures used in the COMBINE study, a research assistant used the timeline follow-back calendar method to assess alcohol intake and the Obsessive Compulsive Drinking Scale to assess craving and administered a symptom checklist and the Profile of Mood States in all groups. Liver function tests and %CDT were measured at weeks 3, 6, 10, and 16. Reasons for early termination were recorded, and full 16-week drinking data were collected where possible.
Statistical Analysis
Group differences in baseline variables, study retention, therapy adherence, and medication compliance were analyzed with analysis of variance or chi-square tests using SAS, version 8.2 (SAS Institute, Cary, N.C.). Participants with at least one postrandomization outcome measurement were included in the efficacy analyses. Two participants in the placebo group, two in the naltrexone-alone group, and two in the naltrexone-gabapentin group did not return for at least one postrandomization evaluation.
Time to the first heavy drinking day was analyzed using Cox regression analysis with percent heavy drinking days at baseline as a covariate and missing data due to dropout censored. A preplanned two-step analysis was conducted evaluating the overall survival curve from study entry through week 16 and the survival curve for the first 6 weeks, when naltrexone and gabapentin were taken together. To evaluate any differential response between those who had a history of alcohol withdrawal symptoms or treatments (those who either experienced alcohol withdrawal symptoms at study entry or had past inpatient detoxification treatment) and those who did not, this variable was entered into the Cox regression analysis along with medication group to evaluate interactions.
Percent heavy drinking days per week and drinks per drinking day were analyzed first over the whole trial and secondarily in the first six weeks (the gabapentin phase) and last 10 weeks (the naltrexone alone phase). If a significant overall effect was found, between-group comparisons were undertaken. Score on the Obsessive Compulsive Drinking Scale and sleep quality were similarly analyzed. The analytic plan used was a linear mixed model evaluating main effects of group, time, and group-by-time interactions (using SAS PROC MIXED).
Alcohol consumption markers (%CDT and γ-glutamyltransferase) were analyzed using the generalized estimating equation approach (PROC GENMOD) across measurements at baseline and at weeks 3, 6, 10, and 16 as binary outcome variables with a positive result indicating heavy drinking. An unstructured covariance matrix was employed.
Results
Participants
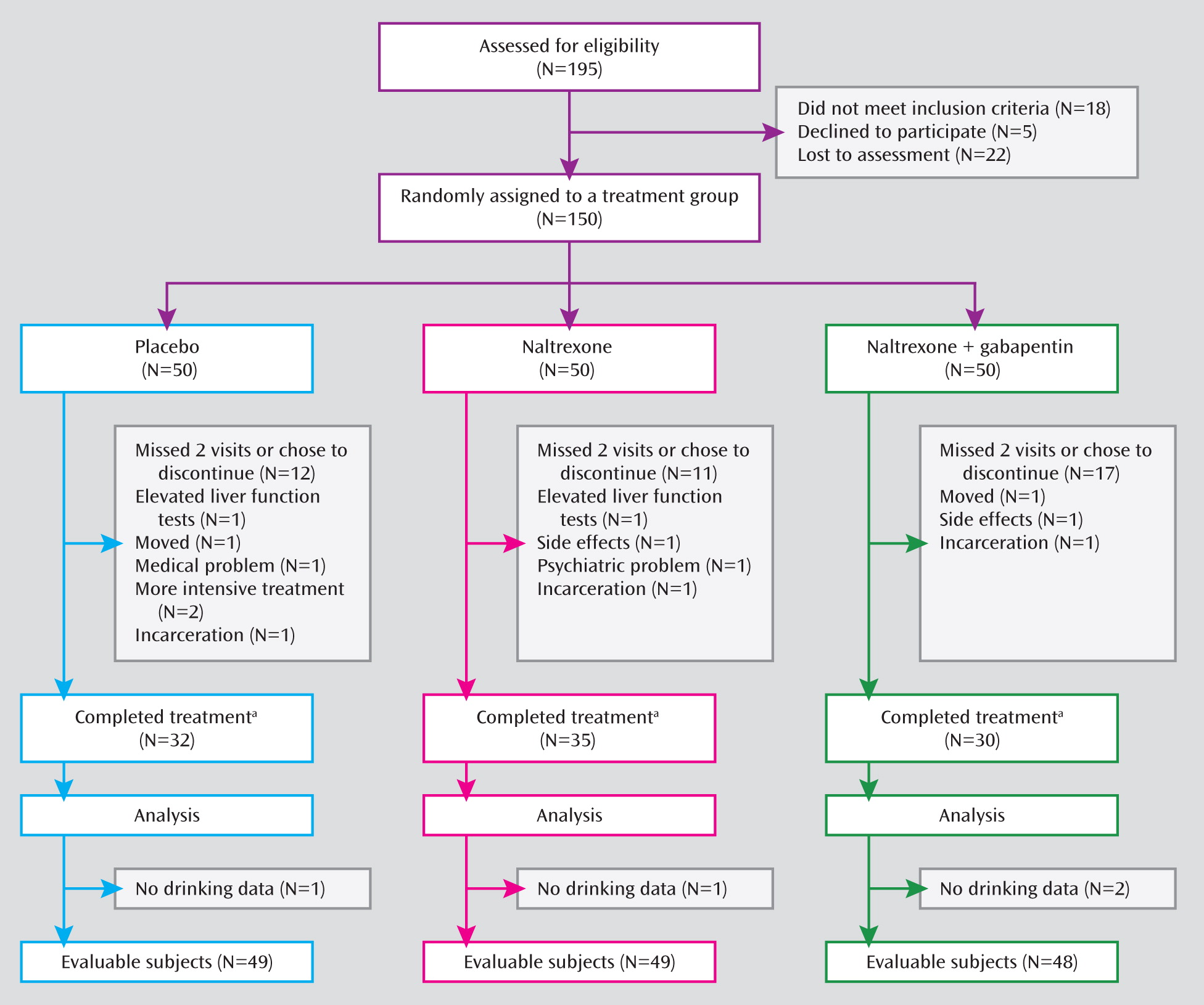
Figure 1 illustrates the process of participant recruitment and randomization. Participant characteristics are summarized in
Table 1. Participants' average age was in the mid-40s, and most were male and Caucasian. On average they drank 12–13 drinks per drinking day on about three-quarters of the days in the 90 days prior to randomization. Of note, 12% of participants were medically detoxified prior to participation in the treatment study (almost exclusively as outpatients by study physicians). There were no differences between medication groups on any demographic or drinking variable.
Retention and Adherence
On average, participants received 10–11 combined behavioral intervention therapy sessions, and the proportion of participants who provided drinking data for all 16 weeks ranged from 82% to 88% in the three groups. Participants took about the same number of medication capsules across all treatment groups (range=92%–96% of prescribed medication). There were no between-group differences.
Drinking Outcomes
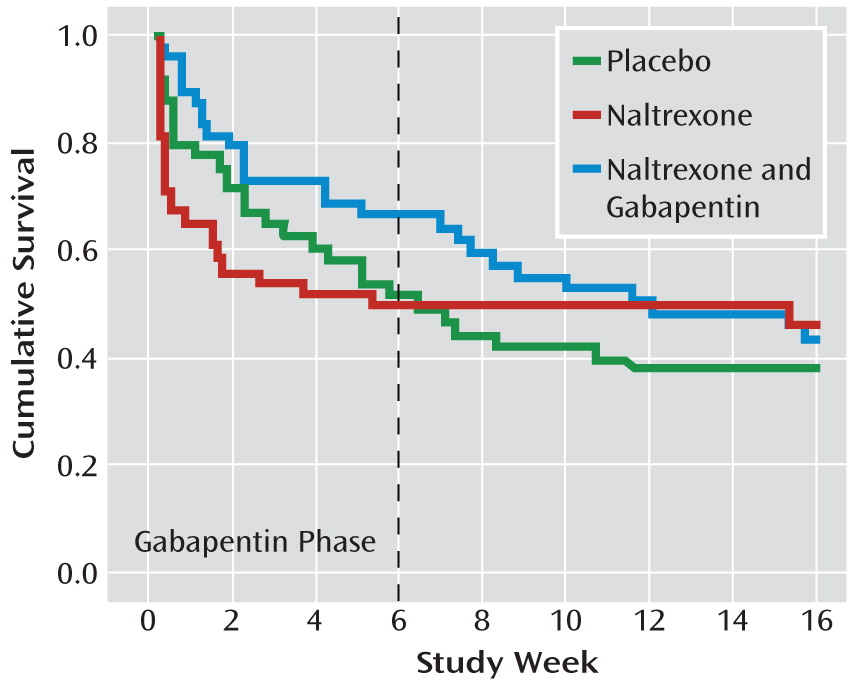
Time to first heavy drinking day is illustrated in
Figure 2. There was an interaction of treatment phase by medication group (p=0.02). During the first 6 weeks, the naltrexone-gabapentin group had a longer time to relapse than the naltrexone-alone group (p=0.04), whose time to relapse was not different from that of the placebo group. However, over the remainder of the trial, there were no treatment group differences.
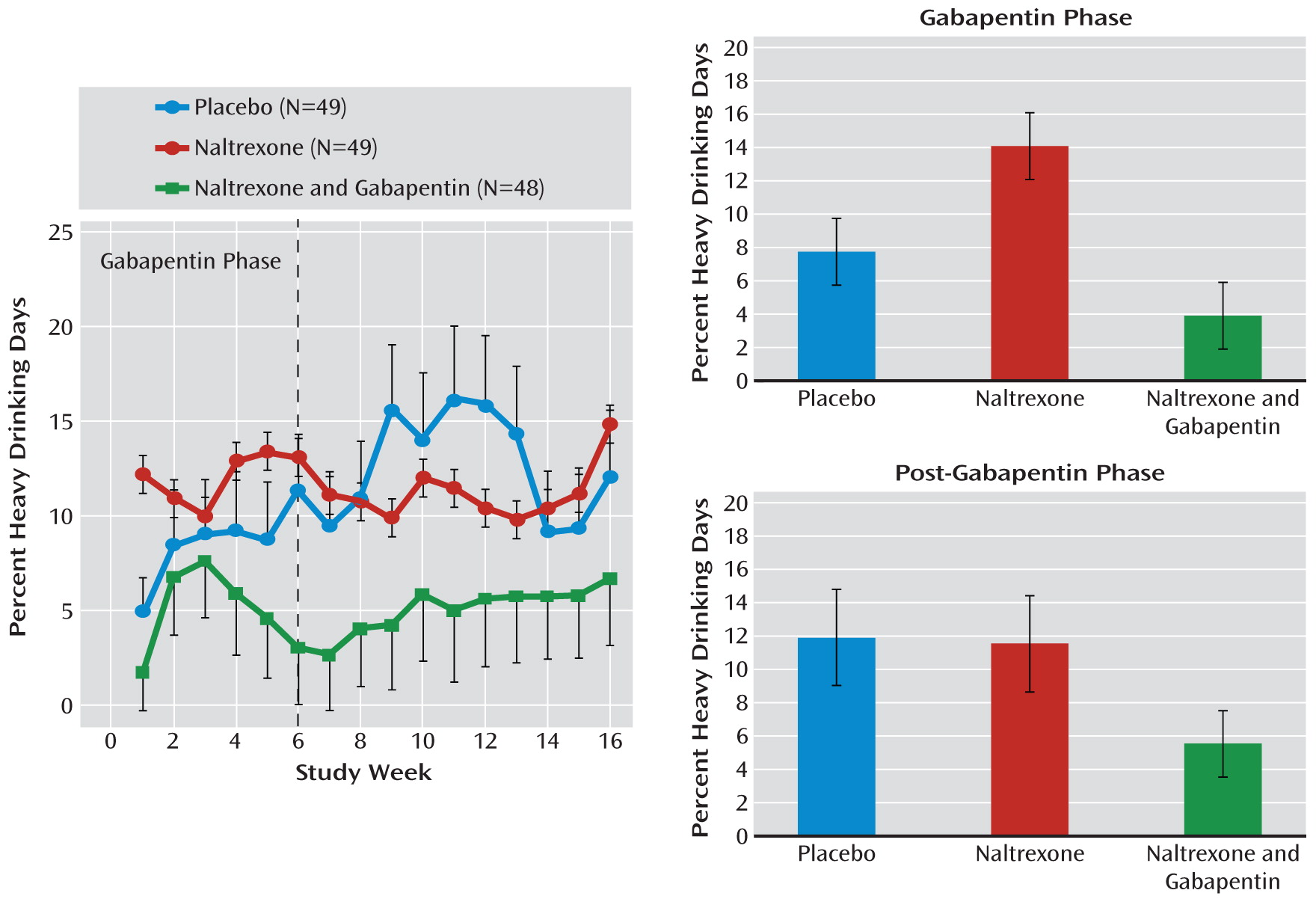
For percent heavy drinking days (
Figure 3), there was a significant difference between medication groups over the 16-week study (F=6.59, df=2, 142, p=0.002). During the first 6 weeks, those treated with naltrexone alone actually did worse than those treated with placebo (t=2.35, df=142, p=0.020), while percent heavy drinking days for the naltrexone-gabapentin group was similar to that of the placebo group (t=1.42, df=142, p=0.160) but significantly lower than that of the naltrexone-alone group (t=1.4, df=142, p<0.001). While the naltrexone-gabapentin group appeared to do better, even after gabapentin was stopped, this difference did not reach statistical significance.
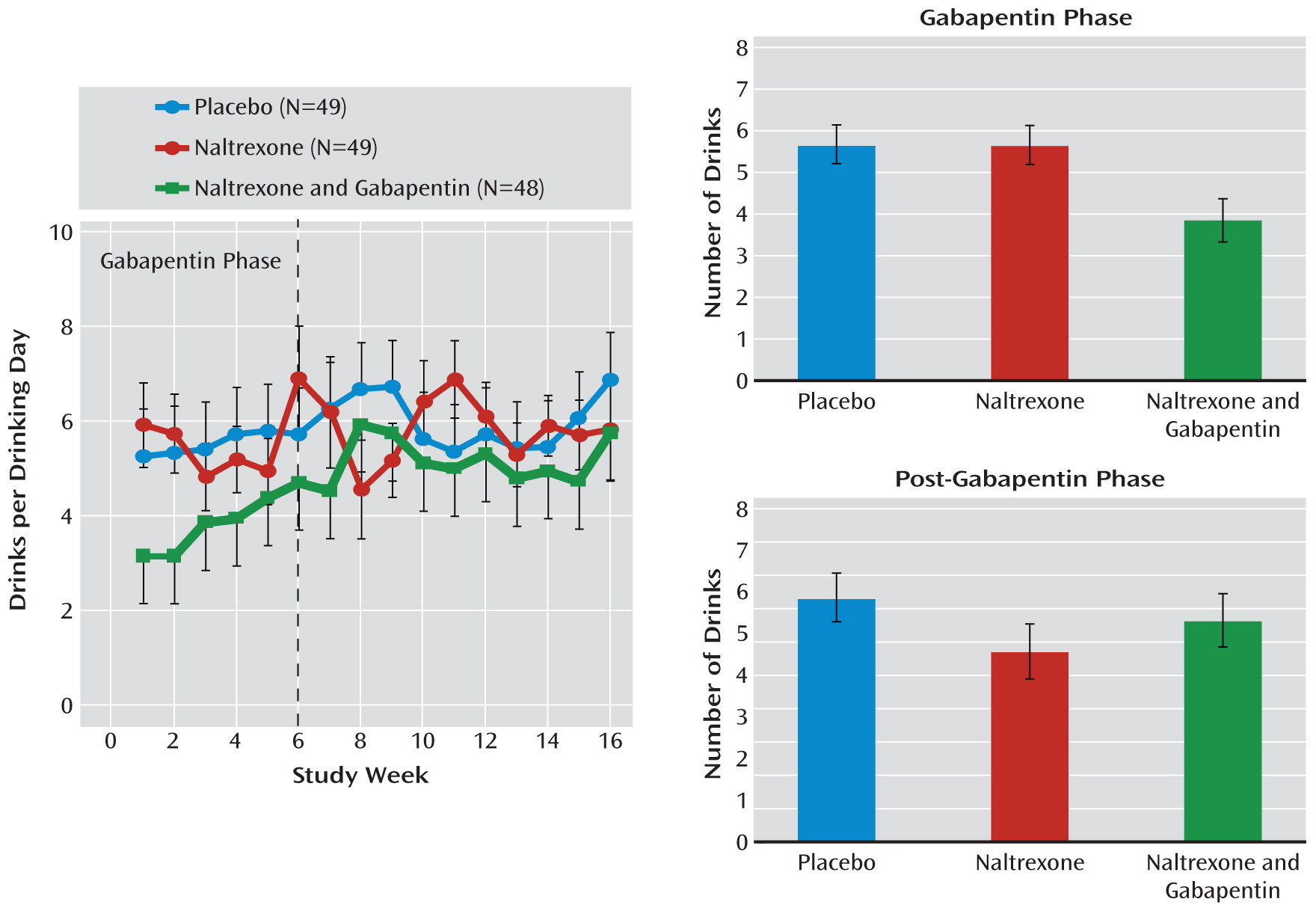
For drinks per drinking day (
Figure 4), there was a significant difference between medication groups over the 16-week study (F=4.77, df=2, 95, p=0.01). During the first 6 weeks, the naltrexone-alone group did not do significantly better than the placebo group, while the naltrexone-gabapentin group did significantly better than both the placebo group (t=2.65, df=81, p=0.01) and the naltrexone-alone group (t=2.57, df=81, p=0.02). After gabapentin was stopped, there were no significant differences between groups.
Craving
There were no significant differences between the groups on the Obsessive Compulsive Drinking Scale score in either phase of the study. On the instrument's resistance control factor subscale, which we previously found to be most responsive to naltrexone (
17), the medication groups differed but not significantly (F=2.33, df=2, 139, p=0.10), with significant differences evident only during the first 6 weeks. There was no significant difference between the placebo and naltrexone-alone groups, but the naltrexone-gabapentin group showed a significantly lower score—that is, more control over drinking urges—than the naltrexone-alone group (t=2.16, df=139, p=0.04) and a lower score than the placebo group but with the difference falling short of statistical significance (t=1.69, df=139, p=0.09).
Biomarkers of Drinking
The probability of having a positive γ-glutamyltransferase test showed a significant group-by-time interaction (χ2=16.8, df=8, p=0.032), with the naltrexone-gabapentin group having significantly fewer positive γ-glutamyltransferase values than the other two medication groups during the gabapentin phase (χ2=7.82, df=2, p=0.02) and fewer positive values after gabapentin was stopped, but falling short of statistical significance (χ2=5.55, df=2, p=0.06).
The probability of having a positive %CDT showed an almost significant group-by-time interaction (χ2=14.7, df=8, p=0.06), with the naltrexone-gabapentin group having significantly fewer positive %CDT values than the other two groups during the gabapentin phase (χ2=6.37, df=1, p=0.012 compared with the naltrexone-alone group and χ2=5.23, df=1, p=0.022 compared with the placebo group). There were no significant differences after gabapentin was stopped.
Overall, the blood tests indicating heavy alcohol consumption were consistent with the verbally reported drinking, showing similar between-group effects.
Sleep Quality, Mood State, and Treatment Response
The difference in sleep quality between groups over the course of the study approached significance (F=2.66, df=2, 140, p=0.07). During the gabapentin phase, there was no significant difference in reported sleep between the placebo and naltrexone-alone groups, but the naltrexone-gabapentin group reported significantly better sleep than did either the placebo group (t=2.49, df=140, p=0.02) or the naltrexone-alone group (t=2.49, df=140, p=0.03).
Poor sleep quality (high scores on the Insomnia Severity Index) was significantly related to heavy drinking, but only in the naltrexone-alone group (B=0.261; SE=0.12, t=2.13, df=139, 0.035)—that is, in the naltrexone-alone group, but not in the other two groups, participants were more likely to drink heavily during periods in which they reported poor sleep.
Profile of Mood States scores or factors were not significantly different between medication groups and did not differentially predict treatment outcomes.
History of Alcohol Withdrawal and Treatment Response
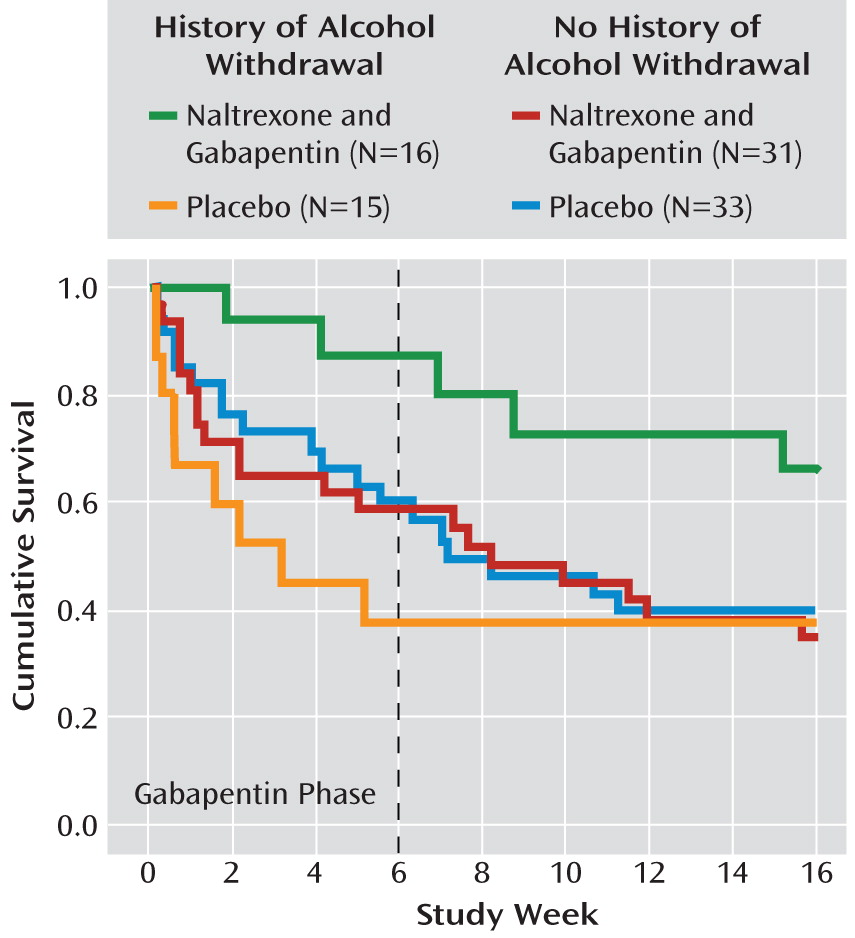
Figure 5 illustrates an overall alcohol withdrawal history-by-medication group interaction (p=0.03). Among participants with a history of alcohol withdrawal, those in the naltrexone-gabapentin group had significantly less relapse to heavy drinking than those in the placebo group (p=0.03), while among participants with no alcohol withdrawal history, there was no difference between these medication groups. An analysis of heavy drinking days during the gabapentin phase essentially found the same relationship. Alcohol withdrawal history had no effect on the comparison between the naltrexone-alone and placebo groups.
Side Effects
Both active medication groups reported more dizziness than did the placebo group (p=0.006). The naltrexone-gabapentin group reported more daytime somnolence than the other two groups (p=0.02) and more blurred vision (p=0.02) and more premature ejaculation (p=0.02) than the placebo group. All these side effects were mild to moderate in severity.
Discussion
As hypothesized, gabapentin, when combined with naltrexone, appeared to be well tolerated and to improve overall efficacy above that noted for naltrexone alone and for placebo. Perhaps surprisingly, naltrexone alone was not superior to placebo in this study, and in fact on some measures it was worse. This is consistent with the results from the COMBINE study (
4), which found that naltrexone added nothing to the efficacy of the combined behavioral intervention. In the present study, combined behavioral intervention was employed as the standard psychosocial intervention and was delivered primarily by the same therapists who delivered it in the COMBINE study at our site. In the COMBINE Study, naltrexone showed superiority over placebo only for those who received medical management without combined behavioral intervention. Since that study was completed after the present one was initiated, we were unaware of this finding and chose to use combined behavioral intervention as an ancillary treatment. Nevertheless, the results of the present study are exactly what would have been predicted by the COMBINE study, that is, that the combined behavioral intervention might mask naltrexone's pharmacological effects. Despite this, it appeared that the naltrexone-gabapentin combination was better than naltrexone alone or placebo on several drinking, craving, and blood-marker outcome variables, especially during the first 6 weeks while participants were actually taking gabapentin. While some of the positive effects of combining gabapentin and naltrexone during the first 6 weeks could still be observed over the next 10 weeks, for the most part these effects were no longer significant, which implies that gabapentin exerted its effects only while participants were actually taking it. We had hypothesized a priori that gabapentin might work best during the initial phases of abstinence, when acute alcohol withdrawal effects might be the most pronounced. The hope was that by using gabapentin to ameliorate symptoms such as insomnia, irritability, and withdrawal craving during this period, naltrexone might have a better chance to work and would continue to work once gabapentin was stopped. This hypothesis could not be validly confirmed.
While we did find an additive response of gabapentin and naltrexone in patients with a history of past or current alcohol withdrawal as well as some favorable effects on sleep while individuals were taking gabapentin, the importance of these findings is not clear. Other researchers found that gabapentin worked better than placebo in preventing relapse in patients who had undergone benzodiazepine detoxification (
10), but they did not compare these patients with patients who had not undergone detoxification. Our group recently reported (
11) that gabapentin worked significantly better than placebo only in those who had clinically significant alcohol withdrawal at study entry. In that study, gabapentin was adjunctive to an initial treatment with intravenous flumazenil, so the direct effect of gabapentin could not be validly confirmed. Group specificity (it worked only in those who had alcohol withdrawal symptoms) and gabapentin's longer-term dosing period (over about 6–7 weeks, similar to the present trial) compared with flumazenil (over 2 days) do imply that gabapentin might have been the primary component of the effective treatment. Of note, in that study, those without current alcohol withdrawal actually did significantly worse on gabapentin compared with placebo, a finding consistent with reports in animal studies (
26). This effect was not observed in the present study. However, the group size of our post hoc exploratory analysis was small, and the definition of alcohol withdrawal history was defined post hoc, limiting the validity of this finding and requiring replication. Nevertheless, the confluence of results suggests that more evaluation of gabapentin by itself is necessary, especially in alcoholics who have current, or possibly past, alcohol withdrawal symptoms and those who do not.
Interestingly, when gabapentin was stopped, there appeared to be some increase in drinking, with worsening in sleep quality (data not shown); the same changes, however, were also noticeable to some degree in the placebo group. It is difficult to determine whether this was a physiological response to discontinuing gabapentin or just an effect of taking fewer pills, especially those at bedtime. In a controlled sleep study, gabapentin improved alcohol-disrupted sleep (
27) and normalized sleep in non-treatment-seeking alcoholics (
28). Our group reported (
29) that gabapentin normalized sleep during alcohol withdrawal better than did lorazepam, but only in patients with a history of multiple detoxifications. Karam-Hage and Brower (
30) originally proposed that gabapentin might work to reduce relapse drinking by “normalizing” sleep, particularly in those who might drink to help with sleep. However, in a more recent study in alcoholic insomniacs (
15), Brower et al. found that while gabapentin delayed onset of heavy drinking after initial abstinence, its efficacy was not attributable to improved sleep.
There was initial concern about the safety of gabapentin, especially when ingested with alcohol. Several controlled studies conducted by us (
31) and others (
14) have largely assuaged that concern. In the present trial, while some low-grade symptoms, such as dizziness, were reported more frequently in the naltrexone-gabapentin group, in general the medication was well tolerated, which is consistent with data from other relapse prevention trials with gabapentin alone (
10,
11). Of note, since gabapentin is excreted in the urine, there would not be any expected interaction with naltrexone on liver metabolism or toxicity, and none was noted in this trial.
Since gabapentin and naltrexone work on different neurophysiological systems, this combination has some appeal. Naltrexone, as an opioid receptor antagonist, appears to reduce the reinforcing aspects of alcohol cues and consumption (
3,
32) while reducing craving and “slip” drinking (
33). Gabapentin, working to normalize GABA and glutamate balance, might work best at restoring normal overall brain activity and tone and be most useful in individuals who have imbalances in these systems, such as those experiencing acute or protracted alcohol withdrawal symptoms. Hence, this medication combination makes pharmacological sense and is consistent with what is known about the neuroscience of addiction in general and of the effects of alcohol on the brain. It is possible, however, since the GABA and glutamate systems also play a role in reinforcement, extinction, and cue-induced learning (
34), that gabapentin plays a primary role in preventing alcohol relapse and reducing drinking, similar to other anticonvulsants, such as topiramate (
35), that have similar basic pharmacological and brain effects. It should be noted, however, that in some pilot work done by our group in a laboratory paradigm designed to test the alcohol antireinforcement effects of medication, gabapentin did not appear to block craving and drinking behavior (
36) in the same way that naltrexone had done previously (
32,
37). However, others have found some effects of gabapentin on alcohol cue-induced craving (
28).
This study had some limitations. It was a single-site study with a limited number of participants. Moreover, participants did not have significant psychiatric conditions other than alcohol dependence, were not on psychiatric medications, were medically stable, and were, for the most part, motivated toward abstinence. Participants received an efficacious psychosocial intervention along with medication and were encouraged to adhere to their medication regimen and the study protocol. In addition, since this study was started prior to knowledge of the potential prediction of naltrexone response by a μ-opioid receptor genetic polymorphism (
38), we could not account for this potential confound. Finally, the independent effect of gabapentin alone could not be evaluated in this study design.
In sum, the addition of gabapentin to naltrexone for the treatment of alcohol dependence seems efficacious and well tolerated. While there are hints that this combination might work best in those who have previously experienced alcohol withdrawal symptoms, further study is needed to confirm this speculation. The data from this study combined with those of others suggest that future studies should explore the use of gabapentin alone while taking into account current or past acute and protracted alcohol withdrawal signs and symptoms, including sleep difficulties and alcohol craving. A better understanding of the role of gabapentin and other anticonvulsants on reinforcement and extinction issues is needed.