Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Study Overview
Site Selection
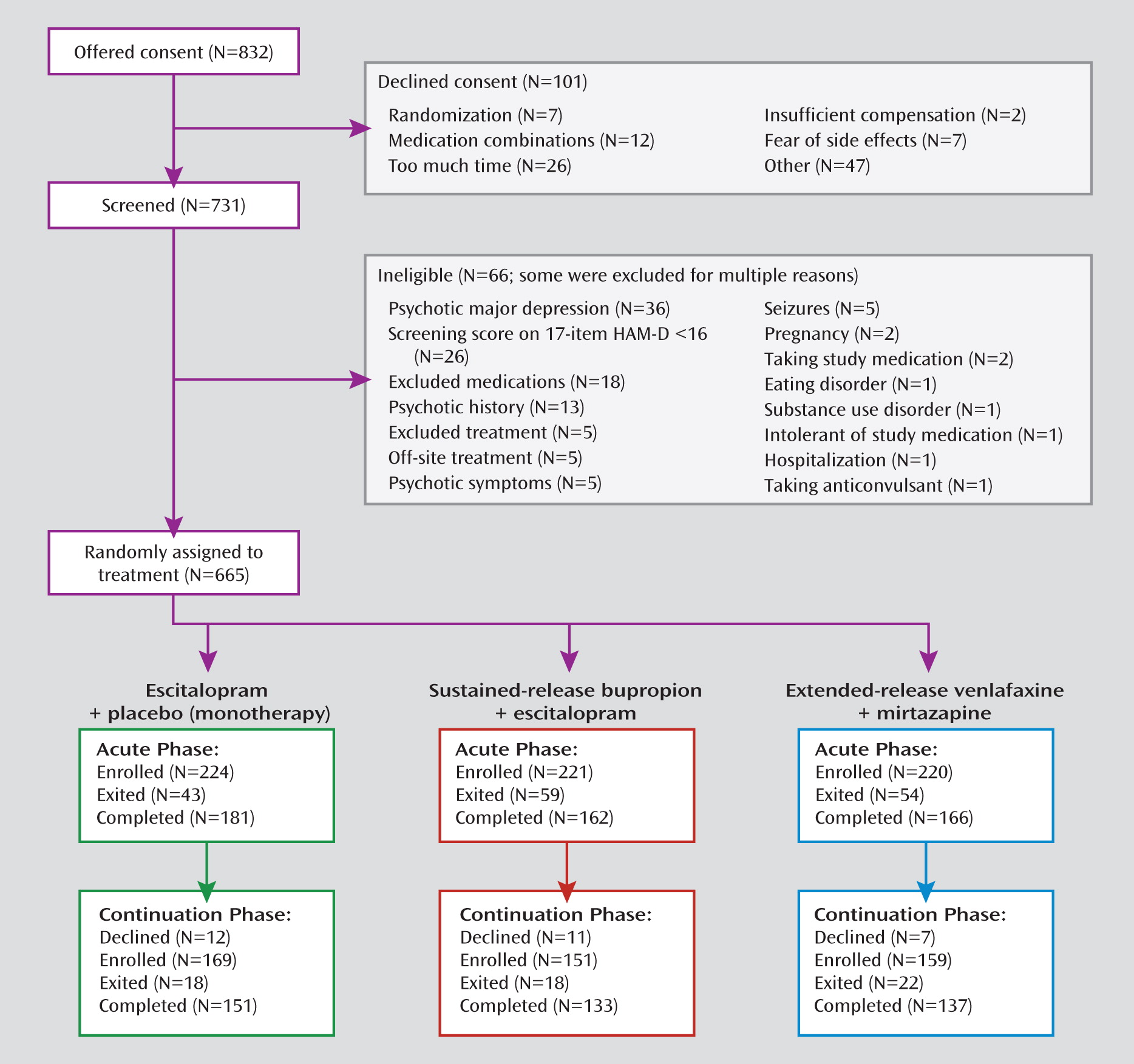
Recruitment
Participants
Baseline Data
Antidepressant Treatment
Escitalopram plus placebo (monotherapy).
Bupropion plus escitalopram.
Venlafaxine plus mirtazapine.
Medication Blinding
Concurrent Treatments
Research Outcomes
Statistical Analyses
Results
Baseline Characteristics
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | Total (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| Sex | 0.43 | <0.05c | ||||||||
| Male | 213 | 32.0 | 81 | 36.2 | 72 | 32.6 | 60 | 27.3 | ||
| Female | 452 | 68.0 | 143 | 63.8 | 149 | 67.4 | 160 | 72.7 | ||
| Race | 0.90 | 0.84 | ||||||||
| White | 431 | 67.0 | 147 | 67.7 | 142 | 67.0 | 142 | 66.4 | ||
| Black | 174 | 27.1 | 56 | 25.8 | 58 | 27.4 | 60 | 28.0 | ||
| Other | 38 | 5.9 | 14 | 6.5 | 12 | 5.7 | 12 | 5.6 | ||
| Hispanic | 101 | 15.2 | 37 | 16.5 | 36 | 16.3 | 28 | 12.7 | 0.95 | 0.26 |
| Employed | 331 | 49.8 | 99 | 44.2 | 119 | 53.8 | 113 | 51.4 | <0.05c | 0.14 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age (years) | 42.7 | 13.0 | 43.6 | 13.1 | 42.4 | 13.5 | 42.1 | 12.4 | 0.34 | 0.22 |
| Education (years) | 13.8 | 3.0 | 13.8 | 3.2 | 13.8 | 2.6 | 13.7 | 3.1 | 0.85 | 0.82 |
| Monthly household income (dollars) | 2,678 | 5,353 | 2,449 | 3,696 | 2,828 | 5,037 | 2,759 | 6,832 | 0.81 | 0.28 |
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| First episode before age 18 | 296 | 44.6 | 96 | 43.0 | 95 | 43.0 | 105 | 47.9 | 0.99 | 0.31 |
| Recurrent depression | 517 | 78.0 | 171 | 76.7 | 174 | 78.7 | 172 | 78.5 | 0.61 | 0.64 |
| Ever attempted suicide | 59 | 9.2 | 14 | 6.5 | 23 | 10.7 | 22 | 10.3 | 0.13 | 0.16 |
| Abused before age 18 | ||||||||||
| Emotionally | 261 | 39.3 | 94 | 42.2 | 88 | 39.8 | 79 | 35.9 | 0.62 | 0.18 |
| Physically | 131 | 19.7 | 45 | 20.2 | 42 | 19.0 | 44 | 20.0 | 0.76 | 0.97 |
| Sexually | 145 | 21.9 | 43 | 19.3 | 50 | 22.6 | 52 | 23.7 | 0.39 | 0.26 |
| Chronic depression (index episode duration ≥2 years) | 368 | 55.5 | 121 | 54.3 | 121 | 54.8 | 126 | 57.5 | 0.92 | 0.49 |
| Chronic or recurrent depression | 0.81 | 0.53 | ||||||||
| Chronic only | 146 | 22.0 | 52 | 23.3 | 47 | 21.3 | 47 | 21.5 | ||
| Recurrent only | 295 | 44.5 | 102 | 45.7 | 100 | 45.2 | 93 | 42.5 | ||
| Both | 222 | 33.5 | 69 | 30.9 | 74 | 33.5 | 79 | 36.1 | ||
| Anxious features (HAM-D) | 497 | 74.7 | 156 | 69.6 | 177 | 80.1 | 164 | 74.5 | 0.02c | 0.25 |
| Atypical features (IDS-C) | 103 | 15.5 | 33 | 14.7 | 38 | 17.2 | 32 | 14.5 | 0.48 | 0.96 |
| Melancholic features (IDS-C) | 124 | 20.5 | 42 | 20.5 | 36 | 18.0 | 46 | 23.0 | 0.53 | 0.54 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age at first episode (years) | 24.0 | 14.1 | 24.4 | 14.4 | 23.9 | 13.7 | 23.7 | 14.2 | 0.85 | 0.53 |
| Years since first episode | 18.7 | 13.6 | 19.3 | 14.4 | 18.5 | 13.4 | 18.4 | 13.1 | 0.69 | 0.76 |
| Index episode duration (months) | 61.7 | 104.8 | 66.4 | 114.4 | 58.1 | 100.8 | 60.6 | 98.5 | 0.91 | 0.77 |
| Scores on clinical ratings | ||||||||||
| HAM-D | 23.8 | 4.8 | 23.4 | 4.9 | 23.8 | 4.6 | 24.3 | 5.0 | 0.34 | <0.05c |
| IDS-C | 38.0 | 9.1 | 37.0 | 8.8 | 37.8 | 9.2 | 39.3 | 9.3 | 0.39 | 0.02c |
| QIDS-C | 15.8 | 3.4 | 15.6 | 3.4 | 15.7 | 3.5 | 16.1 | 3.5 | 0.72 | 0.13 |
| QIDS-SR | 15.5 | 4.3 | 15.2 | 4.0 | 15.3 | 4.6 | 15.9 | 4.2 | 0.77 | 0.10 |
| Altman Self-Rating Mania Scale (39) | 1.5 | 2.3 | 1.6 | 2.4 | 1.6 | 2.2 | 1.3 | 2.2 | 0.79 | 0.21 |
| Cognitive and Physical Functioning Questionnaire (40) | 27.6 | 5.9 | 27.4 | 5.7 | 27.7 | 6.1 | 27.8 | 5.8 | 0.62 | 0.39 |
| Quality of Life Inventory (37) | –1.2 | 1.9 | –1.2 | 1.9 | –1.1 | 1.9 | –1.3 | 1.9 | 0.85 | 0.41 |
| Work and Social Adjustment Scale (36) | 26.9 | 8.8 | 26.2 | 8.8 | 26.7 | 9.2 | 27.9 | 8.4 | 0.50 | 0.04c |
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Illness Variablea | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| N | % | N | % | N | % | N | % | p | p | |
| Comorbid axis I disordersc | ||||||||||
| Agoraphobia | 69 | 10.4 | 20 | 8.9 | 28 | 12.7 | 21 | 9.5 | 0.21 | 0.83 |
| Alcohol abuse | 67 | 10.1 | 23 | 10.3 | 24 | 10.9 | 20 | 9.1 | 0.83 | 0.68 |
| Bulimia | 78 | 11.7 | 27 | 12.1 | 22 | 10.0 | 29 | 13.2 | 0.48 | 0.73 |
| Drug abuse | 35 | 5.3 | 15 | 6.7 | 12 | 5.4 | 8 | 3.6 | 0.58 | 0.15 |
| Generalized anxiety | 131 | 19.7 | 39 | 17.4 | 43 | 19.5 | 49 | 22.3 | 0.58 | 0.20 |
| Hypochondriasis | 29 | 4.4 | 9 | 4.0 | 12 | 5.4 | 8 | 3.6 | 0.49 | 0.84 |
| Obsessive-compulsive | 79 | 11.9 | 27 | 12.1 | 25 | 11.3 | 27 | 12.3 | 0.81 | 0.95 |
| Panic | 65 | 9.8 | 16 | 7.1 | 25 | 11.3 | 24 | 10.9 | 0.13 | 0.17 |
| Posttraumatic stress | 81 | 12.2 | 29 | 12.9 | 32 | 14.5 | 20 | 9.1 | 0.64 | 0.20 |
| Social phobia | 178 | 26.8 | 60 | 26.8 | 59 | 26.7 | 59 | 26.8 | 0.99 | 1.00 |
| Somatoform | 21 | 3.2 | 7 | 3.1 | 7 | 3.2 | 7 | 3.2 | 0.98 | 0.98 |
| Number of comorbid axis I disorders | 0.27 | 0.66 | ||||||||
| 0 | 296 | 44.6 | 107 | 47.8 | 85 | 38.6 | 104 | 47.3 | ||
| 1 | 159 | 23.9 | 51 | 22.8 | 67 | 30.5 | 41 | 18.6 | ||
| 2 | 92 | 13.9 | 27 | 12.1 | 29 | 13.2 | 36 | 16.4 | ||
| 3 | 50 | 7.5 | 16 | 7.1 | 19 | 8.6 | 15 | 6.8 | ||
| ≥4 | 67 | 10.1 | 23 | 10.3 | 20 | 9.1 | 24 | 10.9 | ||
| Number of comorbid axis III disordersd | 0.25 | 0.86 | ||||||||
| 0 | 161 | 24.2 | 55 | 24.7 | 59 | 26.7 | 47 | 21.4 | ||
| 1 | 198 | 29.8 | 66 | 29.6 | 67 | 30.3 | 65 | 29.5 | ||
| 2 | 154 | 23.2 | 54 | 24.2 | 43 | 19.5 | 57 | 25.9 | ||
| 3 | 77 | 11.6 | 20 | 9.0 | 32 | 14.5 | 25 | 11.4 | ||
| ≥4 | 74 | 11.1 | 28 | 12.6 | 20 | 9.0 | 26 | 11.8 | ||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Axis III comorbidity scored | 3.4 | 3.5 | 3.5 | 3.7 | 3.0 | 3.1 | 3.6 | 3.8 | 0.41 | 0.56 |
Outcomes at 12 Weeks
| Patient Groupb | Comparison With Monotherapyc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| 12 weeks | N | % | N | % | N | % | N | % | p | p |
| Weeks in treatment | ||||||||||
| <4 | 93 | 14.0 | 30 | 13.5 | 33 | 14.9 | 30 | 13.7 | 0.66 | 0.94 |
| <8 | 144 | 21.7 | 43 | 19.3 | 55 | 24.9 | 46 | 21.0 | 0.16 | 0.66 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Weeks in treatment | 9.9 | 3.9 | 10.0 | 3.9 | 9.6 | 4.0 | 10.0 | 3.8 | 0.13 | 0.88 |
| Number of postbaseline visits | 5.3 | 2.2 | 5.4 | 2.1 | 5.1 | 2.2 | 5.3 | 2.2 | 0.21 | 0.88 |
| Maximum open dose (mg/day) | — | — | 17.6 | 4.5 | 324.0 | 80.4 | 207.6 | 69.2 | — | — |
| Last open dose (mg/day) | — | — | 16.8 | 5.3 | 287.7 | 121.2 | 192.3 | 82.2 | — | — |
| Maximum blinded dose (mg/day)d | — | — | 1.4 | 0.7 | 14.0 | 7.2 | 25.3 | 32.0 | — | — |
| Last blinded dose (mg/day)d | — | — | 1.3 | 0.7 | 12.5 | 8.3 | 20.0 | 15.7 | — | — |
| 7 months | N | % | N | % | N | % | N | % | p | p |
| Weeks in treatment <12 | 185 | 27.8 | 56 | 25.1 | 72 | 32.6 | 57 | 26.0 | 0.09 | 0.83 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Weeks in treatment | 19.9 | 10.5 | 20.5 | 10.3 | 19.1 | 10.8 | 20.1 | 10.4 | 0.38 | 0.77 |
| Number of postbaseline visits | 7.7 | 3.7 | 7.9 | 3.6 | 7.4 | 3.8 | 7.7 | 3.7 | 0.26 | 0.81 |
| Maximum open dose (mg/day) | — | — | 17.9 | 4.4 | 328.5 | 81.7 | 217.3 | 73.3 | — | — |
| Last open dose (mg/day) | — | — | 15.6 | 6.9 | 271.0 | 136.8 | 177.6 | 94.0 | — | — |
| Maximum blinded dose (mg/day)d | — | — | 1.5 | 0.7 | 14.2 | 7.3 | 26.7 | 32.2 | — | — |
| Last blinded dose (mg/day)d | — | — | 0.7 | 0.9 | 11.5 | 8.6 | 18.0 | 16.4 | — | — |
| Patient Group | Comparison With Monotherapyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | All (N=665) | Monotherapy: Escitalopram Plus Placebo (N=224) | Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | ||||
| 12 weeks | N | % | N | % | N | % | N | % | p | p |
| Early termination | 182 | 27.4 | 55 | 24.6 | 70 | 31.7 | 57 | 25.9 | 0.10 | 0.75 |
| Remissionc | 256 | 38.5 | 87 | 38.8 | 86 | 38.9 | 83 | 37.7 | 0.99 | 0.81 |
| Last QIDS-SR score ≤5 | 242 | 36.6 | 81 | 36.2 | 82 | 37.4 | 79 | 36.2 | 0.78 | 0.99 |
| Reduction in QIDS-SR score ≥50% | 334 | 51.9 | 113 | 51.8 | 111 | 51.6 | 110 | 52.4 | 0.97 | 0.91 |
| Maximum side effect burdend | 0.07 | <0.0001e | ||||||||
| No impairment | 128 | 20.2 | 46 | 21.6 | 44 | 21.0 | 38 | 18.1 | ||
| Minimal/mild | 276 | 43.6 | 110 | 51.6 | 90 | 42.9 | 76 | 36.2 | ||
| Moderate/marked | 167 | 26.4 | 48 | 22.5 | 55 | 26.2 | 64 | 30.5 | ||
| Severe/intolerable | 62 | 9.8 | 9 | 4.2 | 21 | 10.0 | 32 | 15.2 | ||
| Last side effect burdend | 0.66 | 0.64 | ||||||||
| No impairment | 344 | 54.7 | 117 | 55.5 | 118 | 56.5 | 109 | 52.2 | ||
| Minimal/mild | 215 | 34.2 | 74 | 35.1 | 69 | 33.0 | 72 | 34.4 | ||
| Moderate/marked | 53 | 8.4 | 16 | 7.6 | 14 | 6.7 | 23 | 11.0 | ||
| Severe/intolerable | 17 | 2.7 | 4 | 1.9 | 8 | 3.8 | 5 | 2.4 | ||
| At least one serious adverse event | 27 | 4.1 | 8 | 3.6 | 7 | 3.2 | 12 | 5.5 | 0.82 | 0.34 |
| At least one psychiatric serious adverse event | 7 | 1.1 | 1 | 0.4 | 1 | 0.5 | 5 | 2.3 | 1.00 | 0.12 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Last QIDS-SR score | 8.1 | 5.4 | 7.9 | 5.2 | 8.1 | 5.3 | 8.4 | 5.7 | 0.74 | 0.34 |
| Percent change in QIDS-SR score | –45.6 | 35.1 | –46.5 | 35.2 | –44.6 | 34.6 | –45.8 | 35.8 | 0.59 | 0.85 |
| Score on IDS-C anxiety subscale | 2.6 | 2.1 | 2.4 | 2.0 | 2.6 | 2.1 | 2.8 | 2.3 | 0.28 | 0.10 |
| Last Quality of Life Inventory (37) score | 0.2 | 2.3 | 0.1 | 2.4 | 0.3 | 2.1 | 0.1 | 2.4 | 0.57 | 0.92 |
| Last Work and Social Adjustment Scale (36) score | 14.9 | 12.3 | 14.9 | 11.9 | 13.9 | 11.9 | 15.9 | 13.0 | 0.43 | 0.53 |
| Last number of symptom worseningsf | 5.1 | 5.1 | 4.7 | 4.9 | 5.0 | 4.4 | 5.7 | 5.8 | 0.12 | 0.04e |
| 7 months | N | % | N | % | N | % | N | % | p | p |
| Early termination | 244 | 36.7 | 78 | 34.8 | 84 | 38.0 | 82 | 37.3 | 0.49 | 0.60 |
| Remissionc | 298 | 44.8 | 103 | 46.0 | 103 | 46.6 | 92 | 41.8 | 0.90 | 0.38 |
| Last QIDS-SR score ≤5 | 292 | 44.4 | 101 | 45.3 | 101 | 46.3 | 90 | 41.5 | 0.83 | 0.42 |
| Reduction in QIDS-SR score ≥50% | 374 | 58.4 | 129 | 59.4 | 125 | 58.4 | 120 | 57.4 | 0.83 | 0.68 |
| Maximum side effect burdend | 0.14 | <0.0001e | ||||||||
| No impairment | 115 | 18.2 | 43 | 20.2 | 41 | 19.5 | 31 | 14.8 | ||
| Minimal/mild | 269 | 42.5 | 107 | 50.2 | 88 | 41.9 | 74 | 35.2 | ||
| Moderate/marked | 184 | 29.1 | 52 | 24.4 | 60 | 28.6 | 72 | 34.3 | ||
| Severe/intolerable | 65 | 10.3 | 11 | 5.2 | 21 | 10.0 | 33 | 15.7 | ||
| Last side effect burdend | 0.77 | 0.02e | ||||||||
| No impairment | 374 | 59.3 | 135 | 63.7 | 128 | 61.0 | 111 | 53.1 | ||
| Minimal/mild | 184 | 29.2 | 60 | 28.3 | 60 | 28.6 | 64 | 30.6 | ||
| Moderate/marked | 59 | 9.4 | 13 | 6.1 | 15 | 7.1 | 31 | 14.8 | ||
| Severe/intolerable | 14 | 2.2 | 4 | 1.9 | 7 | 3.3 | 3 | 1.4 | ||
| At least one serious adverse event | 46 | 6.9 | 16 | 7.1 | 13 | 5.9 | 17 | 7.7 | 0.60 | 0.82 |
| Last QIDS-SR score | 7.6 | 5.6 | 7.3 | 5.4 | 7.3 | 5.4 | 8.1 | 5.9 | 0.98 | 0.26 |
| Percent change in QIDS-SR score | –49.5 | 36.0 | –50.9 | 34.5 | –49.8 | 37.0 | –47.8 | 36.7 | 0.98 | 0.49 |
| Score on IDS-C anxiety subscale | 2.5 | 2.1 | 2.4 | 2.1 | 2.6 | 2.1 | 2.5 | 2.2 | 0.19 | 0.34 |
| Last Quality of Life Inventory (37) score | 0.5 | 2.4 | 0.4 | 2.6 | 0.6 | 2.1 | 0.4 | 2.4 | 0.27 | 0.87 |
| Last Work and Social Adjustment Scale (36) score | 13.8 | 12.5 | 13.5 | 12.0 | 13.0 | 12.2 | 15.0 | 13.2 | 0.62 | 0.31 |
| Last number of worsening adverse eventsf | 4.9 | 5.3 | 4.7 | 5.4 | 4.8 | 5.2 | 5.4 | 5.3 | 0.47 | <0.05e |
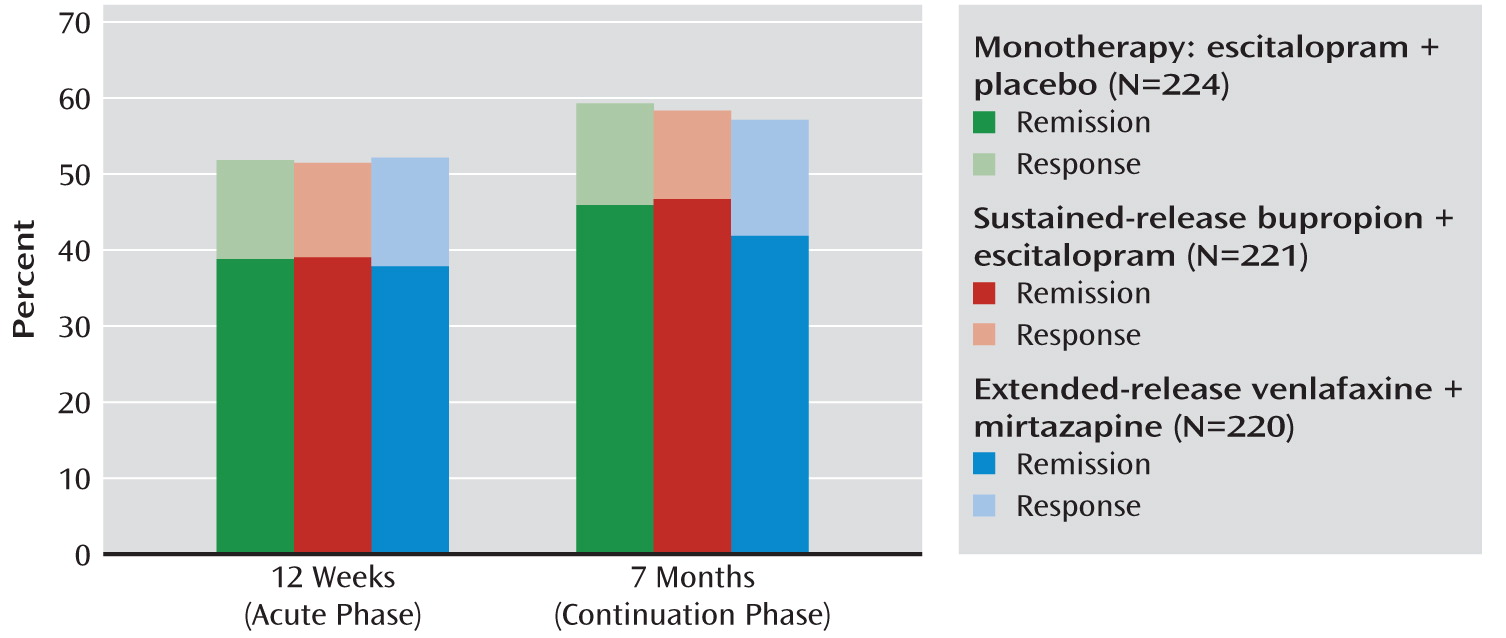
Outcomes at 7 Months
| Comparison with Monotherapy: Escitalopram Plus Placebo (N=224) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sustained-Release Bupropion Plus Escitalopram (N=221) | Extended-Release Venlafaxine Plus Mirtazapine (N=220) | ||||||||
| Outcomea | Unadjusted | Adjustedb | Unadjusted | Adjustedc | |||||
| 12 weeks | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | |
| Early termination | 1.42 | 0.10 | 1.46 | 0.09 | 1.08 | 0.75 | 1.00 | 1.00 | |
| Side effectsd | |||||||||
| Maximum frequency | 1.51 | 0.02e | 1.42 | 0.06 | 2.12 | <0.0001e | 2.05 | <0.0001e | |
| Maximum intensity | 1.82 | <0.001e | 1.73 | 0.003e | 1.97 | 0.0002e | 1.86 | 0.0008e | |
| Maximum burden | 1.37 | 0.09 | 1.28 | 0.18 | 1.96 | 0.0002e | 1.87 | 0.0008e | |
| Last frequency | 1.25 | 0.23 | 1.12 | 0.57 | 1.70 | <0.004e | 1.62 | <0.02e | |
| Last intensity | 1.31 | 0.15 | 1.19 | 0.36 | 1.68 | <0.005e | 1.58 | <0.02e | |
| Last burden | 0.99 | 0.96 | 0.90 | 0.61 | 1.19 | 0.36 | 1.11 | 0.61 | |
| At least one serious adverse eventf | 0.88 | 0.82 | — | — | 1.56 | 0.35 | — | — | |
| At least one psychiatric serious adverse eventf | 1.01 | 1.00 | — | — | 5.19 | 0.14 | — | — | |
| Last QIDS-SR score ≤5 | 1.06 | 0.78 | 1.08 | 0.72 | 1.00 | 0.99 | 1.14 | 0.54 | |
| Reduction in QIDS-SR score ≥50% | 0.99 | 0.97 | 0.96 | 0.85 | 1.02 | 0.91 | 1.03 | 0.87 | |
| Last Work and Social Adjustment Scale (36) scoreg | 0.93 | 0.67 | 0.98 | 0.93 | 1.15 | 0.43 | 0.95 | 0.80 | |
| β | p | β | p | β | p | β | p | ||
| Maximum number of worsening adverse eventsh | 0.07 | 0.26 | 0.08 | 0.19 | 0.11 | 0.06 | 0.11 | 0.06 | |
| Last number of worsening adverse eventsh | 0.07 | 0.46 | 0.06 | 0.54 | 0.20 | <0.04e | 0.14 | 0.14 | |
| Last QIDS-SR score | 0.17 | 0.74 | 0.13 | 0.80 | 0.50 | 0.34 | 0.02 | 0.97 | |
| Percent change in QIDS-SR score | 1.82 | 0.59 | 2.44 | 0.47 | 0.66 | 0.85 | 0.22 | 0.95 | |
| Score on IDS-C anxiety subscale | 0.10 | 0.25 | 0.06 | 0.44 | 0.16 | 0.06 | 0.09 | 0.24 | |
| Last Quality of Life Inventory (37) score | 0.13 | 0.57 | 0.12 | 0.60 | –0.03 | 0.92 | 0.14 | 0.54 | |
| 7 months | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | Odds Ratio | p | |
| Early termination | 1.15 | 0.49 | 1.15 | 0.49 | 1.11 | 0.60 | 1.07 | 0.75 | |
| Side effectsd | |||||||||
| Maximum frequency | 1.53 | <0.02e | 1.44 | <0.05e | 2.31 | <0.0001e | 2.20 | <0.0001e | |
| Maximum intensity | 1.79 | <0.002e | 1.67 | <0.006e | 2.26 | <0.0001e | 2.12 | <0.0001e | |
| Maximum burden | 1.34 | 0.11 | 1.26 | 0.22 | 2.15 | <0.0001e | 2.02 | 0.0002e | |
| Last frequency | 1.40 | 0.08 | 1.32 | 0.16 | 1.80 | <0.002e | 1.76 | <0.004e | |
| Last intensity | 1.53 | <0.03e | 1.48 | <0.05e | 1.94 | 0.0004e | 1.99 | 0.0005e | |
| Last burden | 1.15 | 0.48 | 1.09 | 0.69 | 1.63 | <0.02e | 1.61 | 0.02e | |
| At least one serious adverse event | 0.81 | 0.60 | — | — | 1.09 | 0.82 | — | — | |
| At least one psychiatric serious adverse event | 0.60 | 0.50 | — | — | 1.44 | 0.54 | — | — | |
| Last QIDS-SR score ≤5 | 1.04 | 0.83 | 1.02 | 0.93 | 0.86 | 0.42 | 0.98 | 0.95 | |
| Reduction in QIDS-SR score ≥50% | 0.96 | 0.83 | 0.92 | 0.69 | 0.92 | 0.68 | 0.99 | 0.97 | |
| Last Work and Social Adjustment Scale (36) scoreg | 0.96 | 0.81 | 1.05 | 0.79 | 1.25 | 0.20 | 1.06 | 0.74 | |
| β | p | β | p | β | p | β | p | ||
| Maximum number of worsening adverse eventsh | 0.05 | 0.37 | 0.06 | 0.26 | 0.10 | 0.10 | 0.10 | 0.09 | |
| Last number of worsening adverse eventsh | 0.03 | 0.76 | 0.04 | 0.73 | 0.14 | 0.19 | 0.13 | 0.22 | |
| Last QIDS-SR score | –0.04 | 0.94 | –0.05 | 0.93 | 0.76 | 0.16 | 0.22 | 0.66 | |
| Percent change in QIDS-SR score | 1.14 | 0.74 | 2.09 | 0.54 | 3.17 | 0.36 | 2.12 | 0.53 | |
| Score on IDS-C anxiety subscale | 0.10 | 0.27 | 0.06 | 0.49 | 0.07 | 0.41 | 0.02 | 0.77 | |
| Last Quality of Life Inventory (37) score | 0.26 | 0.27 | 0.24 | 0.28 | –0.04 | 0.87 | 0.11 | 0.64 | |
Discussion
Acknowledgments
Footnotes
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing PsychiatryOnline@psych.org or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

