Autoimmune Diseases and Severe Infections as Risk Factors for Schizophrenia: A 30-Year Population-Based Register Study
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Registers
Study Population
Assessment of Schizophrenia and Other Mental Illness
Assessment of Autoimmune Disease and Infection
| Schizophrenia Spectrum Disorders in Persons Without Infections | Schizophrenia Spectrum Disorders in Persons With Infections | |||||
|---|---|---|---|---|---|---|
| Autoimmune Disease | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| Persons without autoimmune disease (reference) | 1.00 | Reference | 29,372 | 1.60 | 1.56–1.64 | 8,777 |
| Any autoimmune disease | 1.29 | 1.18–1.41 | 483c | 2.25 | 2.04–2.46 | 444c |
| Autoimmune disease with suspected presence of brain reactive antibodies | 1.48 | 1.31–1.68 | 244 | 2.56 | 2.25–2.89 | 243 |
| Autoimmune hepatitis | 2.75 | 1.38–4.83 | 10 | 8.91 | 6.50–11.84 | 43 |
| Autoimmune thyroiditis | 3 | 4.57 | 2.09–8.51 | 8 | ||
| Celiac disease | 2.11 | 1.09–3.61 | 11 | 2.47 | 1.13–4.61 | 8 |
| Guillain-Barré syndrome | 1.22 | 0.58–2.19 | 9 | 2.84 | 1.52–4.76 | 12 |
| Multiple sclerosis | 1.44 | 1.03–1.94 | 39 | 2.10 | 1.37–3.06 | 24 |
| Sjögren's syndrome | 2.07 | 0.82–4.20 | 6 | 4 | ||
| Systemic lupus erythematosus | 1.84 | 0.92–3.23 | 10 | 2.11 | 1.06–3.70 | 10 |
| Thyrotoxicosis (Graves' disease) | 1.94 | 1.47–2.49 | 56 | 2.47 | 1.68–3.49 | 29 |
| Type I diabetes | 1.27 | 1.04–1.53 | 104 | 2.04 | 1.68–2.44 | 109 |
| Other autoimmune diseases | 1.19 | 1.05–1.34 | 256 | 1.95 | 1.70–2.23 | 212 |
| Ankylosing spondylitis | 1.38 | 0.79–2.20 | 15 | 1.68 | 0.72–3.25 | 7 |
| Crohn's disease | 1.22 | 0.88–1.65 | 39 | 1.67 | 1.18–2.27 | 36 |
| Iridocyclitis | 1.32 | 0.87–1.91 | 25 | 1.99 | 1.21–3.06 | 18 |
| Juvenile arthritis | 1.00 | 0.52–1.71 | 11 | 1.77 | 0.95–2.97 | 12 |
| Psoriasis vulgaris | 1.37 | 1.01–1.80 | 47 | 2.77 | 2.07–3.63 | 49 |
| Seropositive rheumatoid arthritis | 0.75 | 0.51–1.06 | 28 | 2.15 | 1.52–2.95 | 35 |
| Ulcerative colitis | 1.22 | 0.97–1.51 | 80 | 1.65 | 1.24–2.14 | 52 |
| Autoimmune diseases with too few cases to calculate individual riskd | 1.59 | 1.13–2.17 | 36 | 2.21 | 1.53–3.07 | 32 |
Data Analyses
Results
| General Population | No Substance Abuse | No Psychiatric Family Historyb | Psychiatric Family Historyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoimmune Disease and Infection | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients |
| Persons without a hospital contact for an autoimmune disease or infection (reference) | 1.00 | Reference | 29,372 | 1.00 | Reference | 22,730 | 1.00 | Reference | 16,041 | 1.00 | Reference | 5,515 |
| Autoimmune disease | 1.30 | 1.19–1.42 | 483 | 1.29 | 1.16–1.43 | 353 | 1.26 | 1.11–1.43 | 238 | 1.16 | 0.93–1.44 | 83 |
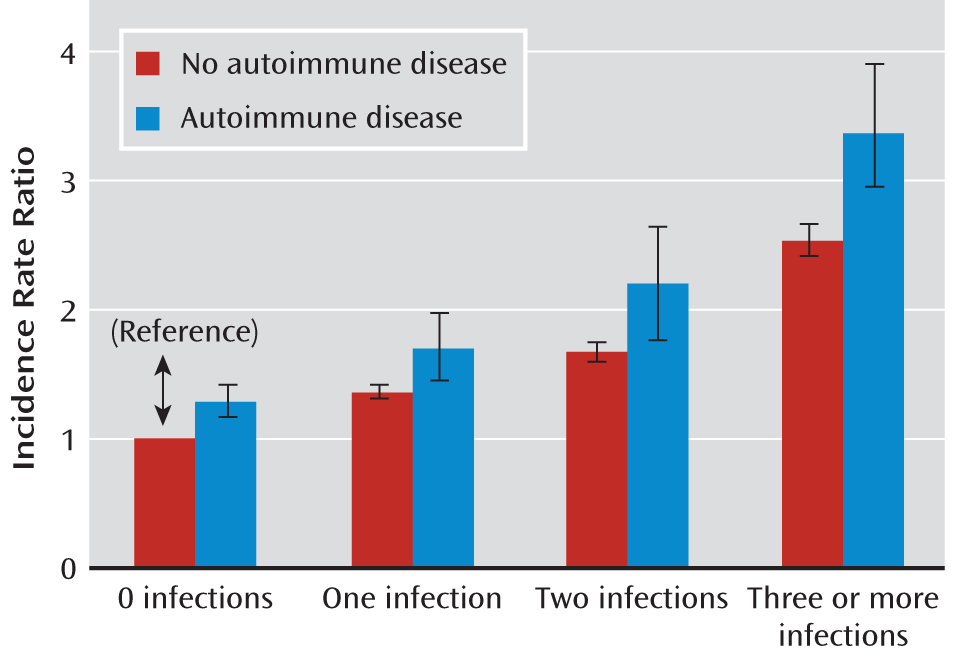
| One infection | 1.39 | 1.35–1.43 | 5,121 | 1.27 | 1.22–1.31 | 3,600 | 1.32 | 1.27–1.37 | 2,908 | 1.23 | 1.15–1.31 | 1,242 |
| One infection and autoimmune disease | 1.73 | 1.48–2.00 | 177 | 1.52 | 1.26–1.82 | 114 | 1.66 | 1.34–2.03 | 91 | 1.27 | 0.88–1.76 | 32 |
| Two infections | 1.70 | 1.62–1.78 | 1,789 | 1.46 | 1.37–1.54 | 1,171 | 1.59 | 1.49–1.69 | 1,007 | 1.41 | 1.28–1.55 | 470 |
| Two infections and autoimmune disease | 2.21 | 1.79–2.68 | 96 | 2.11 | 1.64–2.66 | 66 | 2.26 | 1.70–2.92 | 53 | 1.26 | 0.73–2.02 | 15 |
| Three or more infections | 2.56 | 2.44–2.69 | 1,867 | 1.99 | 1.87–2.11 | 1,070 | 2.33 | 2.18–2.49 | 997 | 1.94 | 1.77–2.12 | 554 |
| Three or more infections and autoimmune disease | 3.40 | 2.91–3.94 | 171 | 2.58 | 2.08–3.15 | 89 | 3.17 | 2.54–3.89 | 86 | 2.79 | 2.03–3.71 | 43 |
| Total | 39,076 | 29,193 | 21,421 | 7,954 | ||||||||
| Infection Only (No Autoimmune Disease) | Autoimmune Disease | |||||
|---|---|---|---|---|---|---|
| Infection | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| Sepsis | 1.95 | 1.47–2.51 | 55 | 4.98 | 2.49–8.73 | 10 |
| Hepatitis | 4.89 | 4.26–5.58 | 212 | 8.89 | 6.03–12.53 | 29 |
| Gastrointestinal | 1.32 | 1.26–1.39 | 1,847 | 1.82 | 1.46–2.24 | 83 |
| Skin | 1.71 | 1.62–1.80 | 1,427 | 2.14 | 1.69–2.66 | 74 |
| Pregnancy-related | 1.14 | 0.98–1.31 | 185 | 1.22 | 0.48–2.47 | 6 |
| Respiratory | 1.53 | 1.46–1.61 | 1,885 | 2.25 | 1.79–2.79 | 77 |
| Urogenital | 1.90 | 1.79–2.01 | 1,200 | 2.70 | 2.10–3.41 | 66 |
| CNS | 1.28 | 1.09–1.50 | 148 | 2.62 | 1.31–4.60 | 10 |
| Other | 1.70 | 1.62–1.78 | 1,818 | 1.99 | 1.60–2.43 | 89 |
| Persons without hospital contact for infection (reference) | 1.00 | 29,372 | 1.30 | 1.18–1.42 | 483 | |
| Infection Only (No Autoimmune Disease) | Autoimmune Disease | |||||
|---|---|---|---|---|---|---|
| Time Since Last Severe Infection | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| <1 year | 2.35 | 2.20–2.51 | 901 | 2.91 | 2.18–3.80 | 50 |
| 1 year | 1.89 | 1.75–2.04 | 670 | 2.85 | 2.09–3.77 | 44 |
| 2 years | 1.73 | 1.59–1.87 | 576 | 2.46 | 1.73–3.37 | 35 |
| 3–4 years | 1.66 | 1.56–1.77 | 1,020 | 2.42 | 1.86–3.08 | 61 |
| 5–9 years | 1.51 | 1.44–1.58 | 1,931 | 2.10 | 1.72–2.54 | 100 |
| 10–14 years | 1.46 | 1.38–1.53 | 1,524 | 2.31 | 1.83–2.87 | 76 |
| ≥15 years | 1.42 | 1.35–1.48 | 2,155 | 1.68 | 1.33–2.08 | 78 |
| Persons without hospital contact for infection (reference) | 1.00 | 29,372 | 1.29 | 1.18–1.41 | 483 | |
Discussion
Strengths and Limitations
Footnotes
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

