Drug dependence is a major contemporary public health issue (
1) involving harmful effects not only for the affected individuals but also for their families, communities, and society as a whole (
2). Community-based family studies indicate that relatives of drug-dependent individuals have eight times the risk of developing drug abuse disorders themselves (
3), but the role of preexisting vulnerability in addiction remains poorly understood. Twin and adoption studies have produced compelling evidence for genetic influences in the development of drug dependence (
4–
7) but have also shown that the high prevalence of drug dependence within families may be caused by environmental factors or interactions between environmental and genetic influences (
8,
9).
The concept of endophenotypes offers a useful strategy for elucidating the underlying factors that render an individual vulnerable to drug dependence. Endophenotypes have been defined as quantitative traits that are intermediate between the predisposing genes (genotype) and the clinical symptoms (phenotype) of a complex disorder. According to the criteria outlined by Gottesman and Gould (
10), endophenotypes are quantifiable traits that are 1) associated with the disorder; 2) genetically determined; 3) largely state independent (i.e., they should manifest in periods of health and during acute illness); 4) cosegregate with the disorder within families; and 5) overrepresented in nonaffected family members relative to the general population. The identification of familial vulnerability markers for drug dependence may provide a scientific basis for the development of effective preventive and therapeutic strategies for individuals at risk.
In our study, we used an endophenotype strategy to identify putative cognitive, emotional, and personality markers of stimulant dependence vulnerability. Addiction is largely subserved by brain circuits that have also been associated with executive control (
11,
12), specifically response inhibition (
13,
14), mental planning (
15,
16), working memory (
17–
19), and attentional control (
20–
22). These neural networks were possibly dysfunctional before the stimulant abuse, rendering individuals vulnerable for addiction. Alternatively, cognitive function may also deteriorate in response to chronic stimulant abuse, a proposal supported by preclinical studies showing that prolonged stimulant abuse leads to deficits in neuropsychological function (
23,
24). Moreover, interactions between both predisposing and neurotoxic effects on cognitive function are equally conceivable.
Stimulant dependence often co-occurs with anxiety and affective disorders (
25,
26), and affect-related psychopathologies are also common in families with substance abuse problems (
27). Depressive symptoms have been associated with both intoxication and withdrawal from various addictive drugs (
28,
29), possibly resulting from drug-induced changes in monoamine neurotransmission (
29,
30). Growing evidence also points to the role of personality traits, attitudes, and demographics in elevating the risk of drug dependence (
31,
32). Both impulsivity (
33,
34) and sensation-seeking (
35) traits have been prospectively associated with a higher risk of drug abuse and addiction. Psychological constructs such as self-efficacy (
36,
37), which describes the confidence in being able to achieve a certain outcome or the perceived control that a person has over life events (
38–
40), may mediate the risk of problem drug abuse.
We compared the cognitive and emotional functioning and the personality traits of stimulant-dependent individuals, their non-drug-dependent siblings, and healthy unrelated comparison volunteers. The rationale for the focus on stimulants is based on relatively high heritability estimates for stimulant dependence (
41). The recruitment of sibling pairs was pragmatically advantageous, but the design has limited power for making inferences about the etiology of personality and cognitive abnormalities evident in both the dependent individuals and their siblings when compared with a twin design. We can say that these represent abnormal familial vulnerability factors (i.e., they are shared by members of the same family), but we cannot discriminate genetic from common environmental causes for their emergence. Nevertheless, the distinction this affords between predisposing factors and drug-induced changes is still valuable. We hypothesized that deficits in executive control function and impulsive personality traits represent an endophenotype for drug dependence and would therefore be identified not only in the drug-dependent individuals but also in their siblings.
Method
Participants
Participants were recruited from treatment services and media advertisements; recruitment figures and participation details are shown in the data supplement that accompanies the online edition of this article. Sibling pairs were included if three conditions were met: 1) they had the same biological parents; 2) one sibling satisfied DSM-IV-TR criteria for cocaine or amphetamine dependence; and 3) the other sibling had no personal history of substance dependence (other than nicotine). All participants who enrolled in the study underwent a screening that included semistructured interviews to ascertain history of drug use, physical health (including signs of acute intoxication and withdrawal), mental health as assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (
42), and demographic characteristics.
Exclusionary criteria, applied for all groups, were a lifetime history of a psychotic disorder or a history of a neurological illness, neurodevelopmental disorder, or traumatic head injury. Healthy comparison volunteers did not have a personal or family history of drug or alcohol dependence. Participants had to be 18–55 years old and able to read and write in English. The study protocol was approved by the Cambridge Research Ethics Committee and written informed consent was obtained from all volunteers before study enrollment.
All drug-dependent individuals (N=50) met DSM-IV-TR criteria for stimulant dependence (94% for cocaine and 6% for amphetamines). The majority (76%) were currently enrolled in a drug treatment program, and all but five were actively using stimulant drugs as verified by urine screens. On average, they had been using stimulants for 16 years (SD=6.4) starting at age 16 (SD=2.8), and they last used them 3 days before testing began (SD=4.7; range=0.5–28 days); without the five drug users who were abstinent, stimulants were last used 1.7 days before testing (SD=2.0), which is consistent with the 72-hour detection window for stimulants. On the Obsessive-Compulsive Drug Use Scale (
43), participants indicated moderate levels of stimulant-related compulsivity (mean score=23.7; SD=9.5). Half of the drug-dependent sample also met DSM-IV-TR criteria for dependence on other substances (54% for opiates, 24% for alcohol, and 8% for cannabis). The drug-taking experiences and the use of alcohol were notably low in the sibling and comparison groups, as reflected by low scores on the Drug Abuse Screening Test (DAST-20;
44) and the Alcohol Use Disorders Identification Test (AUDIT;
45); these results are summarized in
Table 1.
Details about the comprehensive assessment of cognitive function and personality traits are summarized in the online data supplement. The neurocognitive tests were selected on the basis of their known cortico-striatal and medio-temporal neural substrates, which are brain systems that have been associated with the pathophysiology of drug dependence. Personality measures were included because of their hypothesized associations with drug dependence. Measures of trait-anxiety, stress-sensitivity, and trauma history were used as markers of psychological stress or vulnerability. All participants were assessed and tested in a fixed order with the same cognitive battery at a clinical research facility, and they were allowed breaks as needed.
Data Analysis
Data were analyzed using SPSS, version 13, using a five-step strategy:
1.
We performed data reduction by Cambridge Neuropsychological Test Automated Battery (CANTAB) domains to control for the number of tests by calculating summary mean scores from z-transformed test variables within the cognitive domains.
2.
We tested the specificity of cognitive effects by performing repeated-measures analyses of covariance (ANCOVAs) with group as the between-subject factor and domain as the within-subject factor. Where Mauchly tests showed violation of the sphericity assumption, Greenhouse-Geisser corrections were used. Nonsignificant group-by-domain interactions were compatible with the null hypothesis that all cognitive impairments are attributable to deficits in general intelligence.
3.
We used ANCOVA models for group comparisons for each cognitive domain, which were controlled for multiple comparisons using the Bonferroni correction.
4.
We made post hoc comparisons for each significant domain using paired t tests to control for relatedness of siblings and drug-dependent individuals and independent t tests for comparisons between unrelated pairs.
5.
We used permutation statistics for familiality testing for each domain impaired in the sibling pairs relative to healthy comparison volunteers. The within-pair variance between the biological sibling pairs was compared with a permuted distribution of random pairs of drug-dependent-individuals and unrelated siblings (
46).
Additionally, we conducted exploratory analyses at the subdomain level of individual tests using ANCOVA models, followed by post hoc comparisons using the Bonferroni correction. Gender was included as a covariate in all analyses to control for the significant group differences of gender. To obviate the confounding effects of dysphoric mood on cognitive performance (
47), a participant score on the Beck Depression Inventory-II was also included as a covariate in the analysis of the cognitive data. ANCOVA models were applied to compare the results for individuals with and without parental drug or alcohol abuse separately within the drug-dependent and sibling groups; verbal IQ was included as a covariate to control for IQ differences between these subgroups. The affective and personality domains were defined according to the questionnaire constructs, and we followed the same analysis methods as outlined above. Independent t tests were used to compare cognitive and personality profiles of drug-dependent individuals with and without comorbid opioid dependence. The Padua Inventory–Washington State University Revision and the Perceived Stress Scale (version 14) total scores were square-root transformed to reduce skew, as described by Howell (48). For the group differences in demographic characteristics, ANCOVA models, chi-square tests, or Fisher’s exact tests were applied as appropriate for the analysis of categorical data. Pearson correlations were estimated where appropriate using the Bonferroni correction. All tests were two-tailed, and we set a significance level of 0.05. Because of technical problems, Rapid Visual Information Processing, One-Touch Stockings of Cambridge, and stop-signal data for one drug-dependent participant were unavailable.
Results
Demographic, Familial, Social, and Mood Data
The groups were matched for age, verbal intelligence, and duration of formal education (
Table 1), but they differed with regard to gender, as the majority of drug-dependent individuals were men. The shared familial environment in the sibling pairs, which lasted in most cases into adolescence, was notably different from the childhood familial environment reported by healthy volunteers; the sibling pairs had larger household sizes, a higher incidence of parental divorce and of parents with addiction problems, and more frequent experiences of childhood abuse.
Cognitive Domains
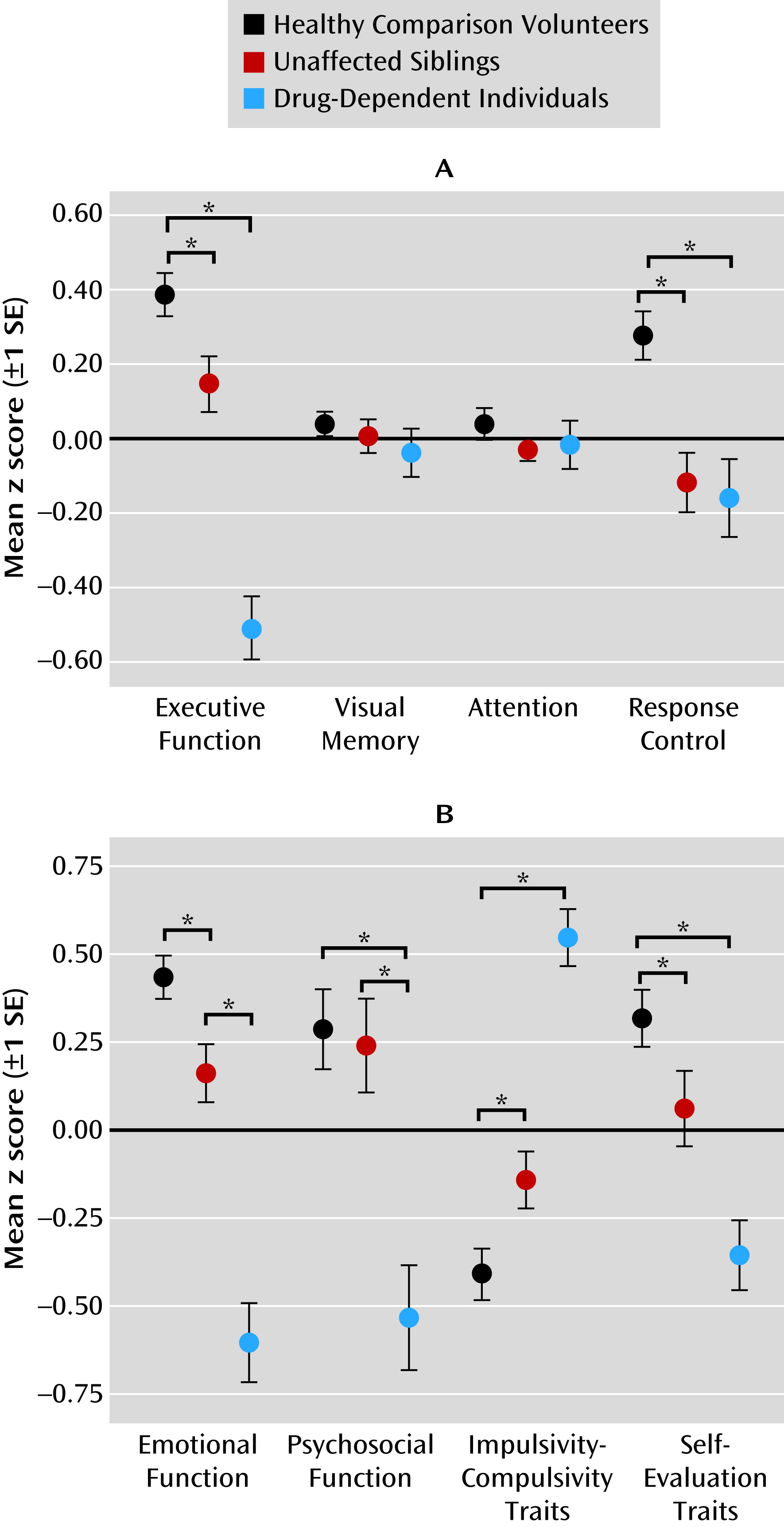
Task performance differed significantly over the four cognitive domains (executive function, visual memory, attention control, and response control) across the three groups, as reflected by a highly significant domain-by-group interaction (F=6.7, df=2.6, 371.7, p<0.001), refuting the null hypothesis that all cognitive impairments were attributable to deficits in general intelligence. No significant main effects of domain were found, but there was a significant main effect of group (F=9.6, df=2, 142, p<0.001). To investigate the nature of the interaction, we compared the groups separately on each domain, revealing significant results for the domains of executive function (F=16.0, df=2, 144, p=0.004) and response control (F=5.9, df=2, 144, p=0.012).
Figure 1A shows that the difference between groups in terms of visual memory and attention did not reach significance. Post hoc comparisons revealed that both siblings and drug-dependent individuals differed significantly from healthy volunteers in executive function (t
sibs=2.6, p=0.012; t
drug=8.8, p<0.001) and from each other (t=5.9, p<0.001). In response control, the sibling pairs differed significantly from healthy volunteers (t
sibs=4.0, p<0.001; t
drug=3.6, p<0.001) but not from each other. Parental drug or alcohol abuse was associated with aggravated performance in executive function and visual memory only in drug-dependent individuals but not in their siblings.
Affective and Personality Domains
We observed a significant domain-by-group interaction for affective and personality domains (F=23.0, df=2, 45, 356, p<0.001), providing support for the hypothesized specificity of the domains. We observed a significant main effect of group (F=10.2, df=2, 45, 356, p<0.001) but not a significant main effect of domain. Group comparisons conducted separately for each domain revealed significant group differences in all four domains: emotional (F=37.3, df=2, 145, p<0.001), psychosocial (F=8.7, df=2, 146, p<0.001), impulsivity-compulsivity (F=36.7, df=2, 146, p<0.001), and self-evaluation (F=11.8, df=2, 146, p<0.001). As shown in
Figure 1B, both siblings and drug-dependent individuals differed significantly from healthy comparison volunteers in emotional functioning (t
sibs=2.8, p=0.007; t
drug=8.4, p<0.001), impulsivity-compulsivity (t
sibs=−2.6, p=0.012; t
drug=−8.9, p<0.001), and self-evaluation traits (t
sibs=2.0, p=0.044; t
drug=5.3, p<0.001). On psychosocial functioning, the siblings were not different from healthy volunteers, but data from drug-dependent individuals revealed significant psychosocial impairment (t=4.4, p<0.001).
Figure 1B further illustrates that the impairments in all four domains were exacerbated in the drug-dependent individuals compared with their siblings (emotional: t=−5.5; psychosocial: t=−3.4; impulsivity-compulsivity: t=6.5; self-evaluation: t=3.2; p<0.005 in all cases). Parental drug or alcohol abuse did not affect emotional function and personality traits in the sibling pairs.
Familial Relatedness and Traumatic Childhood
The difference between sibling pairs and healthy volunteers reached significance in executive function and response control. We compared the within-pair variance between the biological sibling pairs in these two measures with the variance between random sibling pairs (
46). The observed variance on response control was significantly smaller within the biological pairs compared with the randomly permutated distribution (p=0.006), indicating that impairment in response control is a shared trait between family members. For executive function, variances in biological pairs did not significantly differ from those in random sibling pairs, suggesting that impairments in executive function are not familial. Biological sibling pairs also differed significantly from unrelated healthy volunteers in emotional function, impulsivity-compulsivity traits, and self-evaluation, but the variances within these biological pairs did not significantly differ in any of these domains from variances within randomly permutated pairs. Comorbid dependence on opioids was not related to a different cognitive or personality profile in the current sample.
The sibling pairs reported significantly higher levels of childhood trauma compared with unrelated healthy volunteers (
Table 1). We used a composite variable across all of the three types of abuse (
49) to explore the relationship within the sibling pairs’ traumatic childhood and the cognitive and personal domain measures. Executive function (r=−0.3, p<0.05) and impulsive-compulsive traits (r=0.3, p<0.05) were significantly associated with the degree of childhood abuse in the sibling pairs. The significant relationship between childhood abuse and emotional function, however, did not survive correction for multiple comparisons.
Cognitive Profiles and Indexes of Drug Abuse
Correlational analyses between last stimulant use, duration of stimulant abuse, and cognitive or personality domain measures did not reach significance. Compulsive pattern of stimulant use was significantly associated with emotional functioning (r=−0.5, p<0.05) and self-evaluation (r=0.5, p<0.05). Relationships between alcohol use (as reflected by the AUDIT score) and cognitive/personality measures did not survive correction for multiple comparisons. No statistically significant relationships were observed between cognitive performance and the duration of cannabis use or the age at onset. Trait impulsivity and anxiety traits were associated with each other in all volunteers (r=0.5, p<0.05). Cognitive and personality domains correlated with each other; a full correlation matrix can be found in the online data supplement.
Discussion
By comparing the characteristic phenotypes of drug-dependent individuals with those of their unaffected siblings and unrelated healthy volunteers, our study identified candidate endophenotypes for drug dependence. We found shared abnormalities in the sibling pairs in domains of executive cognitive and response control, emotional function, impulsivity-compulsivity traits, and self-evaluation; the detailed results are summarized in
Table 2.
Cognitive Endophenotypes for Drug Dependence
The siblings’ cognitive profile was characterized by deficits in executive function such as working memory and mental planning. The impairments became especially apparent when the rapid suppression of an ongoing, well-established response was required. The siblings showed a general slowing in response speed, and this was even slower in drug-dependent individuals (
Table 2). Similar profiles of impaired response regulation have been reported in adults with a family history of alcohol dependence (
50) and in children with drug-dependent parents (
51,
52). We believe that the cognitive deficits identified in both drug-dependent individuals and their siblings represent a shared trait in family members that predates the exposure to stimulant drugs and may be a predisposing risk factor for the development of drug dependence. This proposal is supported by a longitudinal study in boys with and without a family history of alcoholism: poor inhibitory control, as indicated by prolonged stop-signal response time, predicted the onset of substance abuse in all children, but most strongly in those with alcohol-dependent parents (
53).
Contemporary models of the pathogenesis of drug dependence suggest that immaturity of the regulatory control systems in the prefrontal cortex renders adolescents vulnerable to the initiation of drug abuse (
54). The progressive breakdown of inhibitory control implemented by this circuitry, however, has been attributed to the repeated abuse of drugs, possibly paving the way for the development of drug dependence (
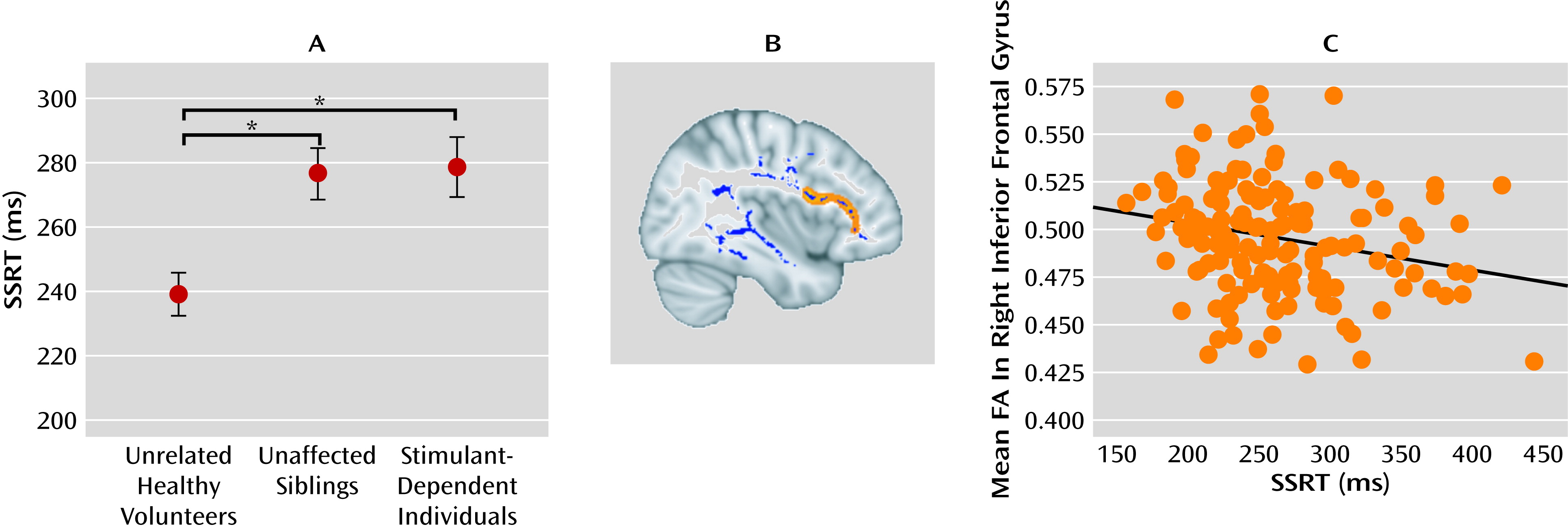
55). Impaired regulatory abilities in those siblings without a history of chronic drug abuse might indicate a developmental dysfunction of prefrontal control. We recently showed that impaired stop-signal performance in the nondependent siblings of drug-dependent individuals was associated with reduced fractional anisotropy in diffusion tensor imaging scans of frontal white matter, which is compatible with disrupted anatomical connectivity of the inferior prefrontal cortex (
56) (see
Figure 2). The low within-pair variation in response control performance between the sibling pairs further supports the familial risk for impaired inhibitory control in people with stimulant dependence and their first-degree relatives. Yet, despite this higher familial risk of addiction, some of the siblings have experimented with drugs but never made the transition to addiction. Thus, occasional exposure to drugs, such as the recreational use of cannabis and social drinking, did not produce addiction in these high-risk individuals. The potentially protective factors against addiction in at-risk individuals are incompletely characterized and merit further study.
Personality Endophenotypes for Drug Dependence
As shown by our preliminary data, the sibling pairs reported significantly higher levels of trait impulsivity than did the healthy comparison volunteers (
57). Our data further indicate reduced emotional functioning in the sibling pairs, as reflected by high levels of trait anxiety and stress sensitivity (
Table 2). Anxious-impulsivity trait has previously been described by Newman and Wallace (
58) as a breakdown of inhibitory control in situations when the escape from aversive consequences appears impossible. More recently, a similar concept of
negative urgency has been proposed that describes a personality trait of impulsive actions in response to intense negative affect (
59). The higher levels of anxious-impulsivity in the sibling pairs suggest underlying deficits in emotion regulation, an ability that develops through the maturation of the fronto-limbic brain systems (
60,
61). Developmental imbalances between these systems generally emerge during adolescence. Emotion systems in limbic structures mature before control systems in the prefrontal cortex (
60), possibly laying the foundation for individual differences in emotion regulation or psychopathologies (
62,
63). The concept of self-evaluation includes individuals’ beliefs and perceptions about themselves that influence their mental and social well-being (
64). Low levels of self-efficacy and self-perception in relation to other people, as shown in the present sample (
Table 2), have been associated with addictive behaviors (
65).
Relationships between childhood trauma and executive function as well as impulsivity have been reported in stimulant-dependent individuals (
66). These relationships are not just restricted to dependent individuals but also affect their nondependent siblings. Our results are therefore consistent with the contemporary literature, suggesting that abuse experiences during childhood have long-lasting effects on cognitive function and behavior in adulthood (
67–
69).
Dysfunction Associated With Chronic Drug Abuse
As hypothesized, and in keeping with previous studies (
15,
18,
70), cognitive function in drug-dependent individuals was significantly impaired on all tests. Impairments in executive function were significantly exacerbated in drug-dependent individuals compared with their siblings, suggesting that neurotoxic or other ancillary effects of chronic drug abuse may account for the scale of the impairments. However, we did not find significant correlations between the duration of stimulant abuse and cognitive performance, indicating that the relationship is more complex than for a simple exposure hypothesis. The fact that almost all drug-dependent individuals tested positive for stimulants at the time of testing may also have affected performance to some degree (
71,
72). We did not find any relationships between the time of last stimulant use and performance in the measured cognitive domains. Although chronic drug abuse has a negative influence on cognitive performance, some cognitive deficits also exist in first-degree relatives in the absence of drug abuse. Cognitive dysfunction may thus be considered as a stable trait predating drug-taking that is independent of the states of intoxication or withdrawal.
Consistent with our preliminary data (
57), higher levels of sensation- and reward-seeking personality were observed only in drug-dependent individuals but not in their siblings (
Table 2), suggesting that the need for excitement is specific for individuals with chronic stimulant drug exposure. Levels of anxious-impulsivity were also higher in drug-dependent individuals than in their siblings, indicating that chronic drug abuse further increases both anxiety and impulsive traits. The significantly higher levels of stress-sensitivity in drug-dependent individuals are consistent with the notion that the system implicated in stress overlaps with brain circuitries associated with addiction (
73,
74).
Strength and Weaknesses
The strengths of the study include the relatively large sample size and well-characterized participants in terms of personality and demographic variables. The neuropsychological tests we used have been validated in terms of their neural substrates (
75). The test battery was theoretically focused on cognitive mechanisms of special relevance to drug dependence and demonstrated excellent sensitivity for the detection of cognitive endophenotypes in sibling pairs. The drug-dependent individuals were recruited from a variety of sources, enhancing the generalizability of the results. From its relatively novel perspective, our study confirmed and extended several findings using other approaches, suggesting that male gender, stressful life events, dysfunctional family structure, anxious personality, and impulsivity are associated with a heightened risk for drug dependence (
32). Although the siblings shared many of these risk factors with their drug-dependent brothers and sisters, they did not engage in drug abuse. Their average age of 33 years suggests that they may have had many opportunities to initiate drug abuse and develop dependence, which makes them ideal candidates for the study of endophenotypes.
Potential limitations of the study include the use of a sibling design instead of a twin design; the latter would have allowed the disentangling of genetic from environmental influences, but it would have been prohibitively difficult to complete. However, we used permutation statistics to test the familiality of salient markers as well as correlational analyses to explore the relationships with childhood trauma and the candidate endophenotype markers. Longitudinal studies are now warranted in order to clarify whether abnormalities that are
not shared between the sibling pairs reflect a more selective higher “dose” of genetic or environmental risk factors in those individuals who become dependent. In contrast to previous research suggesting that the genetic influence on opioid abuse is specific and not shared with other drugs (
8), we did not find differences in the cognitive and personality profiles of drug-dependent individuals with and without opiate dependence or between their siblings. However, further research is also needed to clarify whether these findings reflect a specific vulnerability to stimulants or to drug dependence more generally. Finally, our study does not directly address the important question of why some individuals with familial risks do not become dependent on drugs. This shortcoming lies in the endophenotype concept itself, which defines “intermediate markers of genetic risk,” not of resilience. Presumably, the nondependent siblings have benefited from some resilience or protective factors to offset the risks they shared with their dependent siblings, but it is not clear what these protective factors might be.
Acknowledgments
The authors thank all the volunteers, the staff at the Mental Health Research Network, the staff at the Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital, and Daisy Appanah for her invaluable help in making room at the Research Facility available whenever needed.