The standard antidepressants, including selective serotonin reuptake inhibitors (SSRIs), are generally regarded as safe and effective treatments. However, more than a few weeks may be required to achieve reliable antidepressant responses (
1,
2). From a clinical perspective, a more rapid treatment response would be important in treating depressed patients not only because of the high morbidity during the latent period before the onset of antidepressant effects (
2–
4) but also because of the association between faster response and better long-term outcome (
5). Moreover, in light of the results from a large-scale trial indicating that the response and remission rates of depressed patients treated with standard antidepressants, such as citalopram, were relatively modest (
6), efforts to find novel therapeutic approaches that can yield greater efficacy, as well as more rapid responses, would be valuable.
Creatine monohydrate (creatine) has been established as a safe natural product and has been available as a dietary supplement for more than a decade (
7,
8). We found that oral supplementation with creatine increased the cerebral reservoir of phosphocreatine (
9). An increased brain creatine reservoir may cause a shift in brain creatine kinase activity and ultimately be used to produce adenosine triphosphate (ATP) from phosphocreatine in response to energy demand (
9–
11). Previous studies using magnetic resonance spectroscopy with phosphorus-31 (
31P-MRS) have suggested that subjects with major depressive disorder have abnormal brain bioenergetics (
12–
15) and that as symptoms improve with treatment, altered turnover of ATP to phosphocreatine is normalized (
12–
16). Furthermore, a high phosphocreatine level and low level of β-nucleoside triphosphate, resonance of which comes mainly from ATP in the brain, at baseline were associated with a subsequent positive treatment response to an SSRI and triiodothyronine augmentation (
13). This suggests that the proenergetic effect of creatine supplementation, including efficient regeneration of intracellular high-energy phosphate, may contribute to an earlier and greater response to standard antidepressants.
Recent preclinical evidence from an animal model of depression also provides support for the potential antidepressant effects of creatine (
17). This antidepressant-like response induced by creatine supplementation was observed in female, but not male, rodents. Although it is not clear why the antidepressant efficacy of creatine supplementation was predominantly in female rodents, sexual dimorphism in expression of creatine kinase levels (
18) and beneficial effects of estrogen on mitochondrial function (
19,
20)—for example, greater energy-producing capacity and lower reactive oxygen species production—could play a role in these sex-dependent creatine effects in animal models. Furthermore, abnormal cerebral metabolism associated with depression has been reported to be more common in women than in men (
12), suggesting that creatine supplementation may be more beneficial in women. On the basis of these preliminary findings, we focused on women in what we believe is the first double-blind placebo-controlled study of creatine augmentation for major depressive disorder.
In this clinical trial, the efficacy, safety, and tolerability of creatine augmentation of escitalopram were assessed in individuals with major depression. Given the nature of the proof-of-concept study, only depressed women were selected as study subjects because creatine’s antidepressant-like effects have been shown preclinically only in female animals. We expected that creatine augmentation would be associated with earlier and greater improvements in depressive symptoms than placebo augmentation.
Results
Study Group
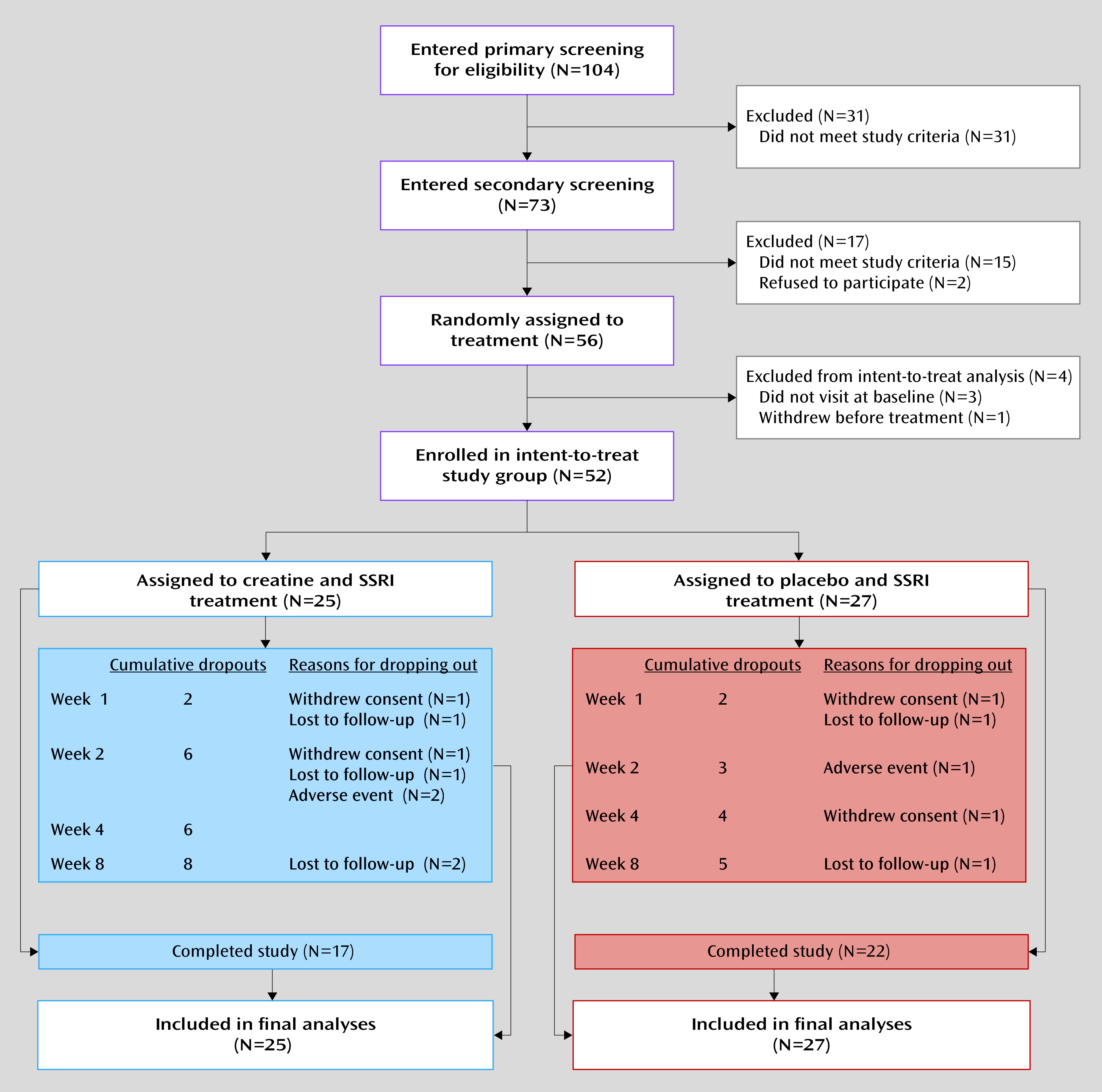
Of the 52 enrolled women with major depression, 25 (48.1%) were randomly assigned to receive creatine augmentation of SSRI and 27 (51.9%) were assigned to placebo augmentation (
Figure 1). Baseline demographic and clinical characteristics were similar in the two treatment groups, as shown in
Table 1.
A total of 39 patients (75.0%) completed the trial. There was no significance difference in the study dropout rates between treatment groups: 32.0% in the creatine group (N=8) and 18.5% in the placebo group (N=5) (χ
2=1.26, df=1, p=0.26). Reasons for discontinuation were withdrawal of consent and loss to follow-up (
Figure 1). Two patients in the creatine group and one patient in the placebo group discontinued treatment at week 2 because of adverse events.
Adherence to treatment medication, which was measured by the self-administered visual analogue scale at each visit, was comparably high in the two groups (self-reported average adherence rate, creatine: 95.1%; placebo: 96.0%) (p=0.62). The dosage of escitalopram did not differ between treatment groups at any visit; the overall mean dose was 15.0 mg/day (SD=3.5) in the creatine group and 15.7 mg/day (SD=2.9) in the placebo group (p=0.43). Concomitant use of lorazepam was reported for 10 patients with creatine augmentation (40.0%) and 12 patients with placebo augmentation (44.4%) (p=0.75). Zolpidem was prescribed for six patients in the creatine group (24.0%) and nine patients in the placebo group (33.3%) (p=0.46).
Primary Outcome Measure
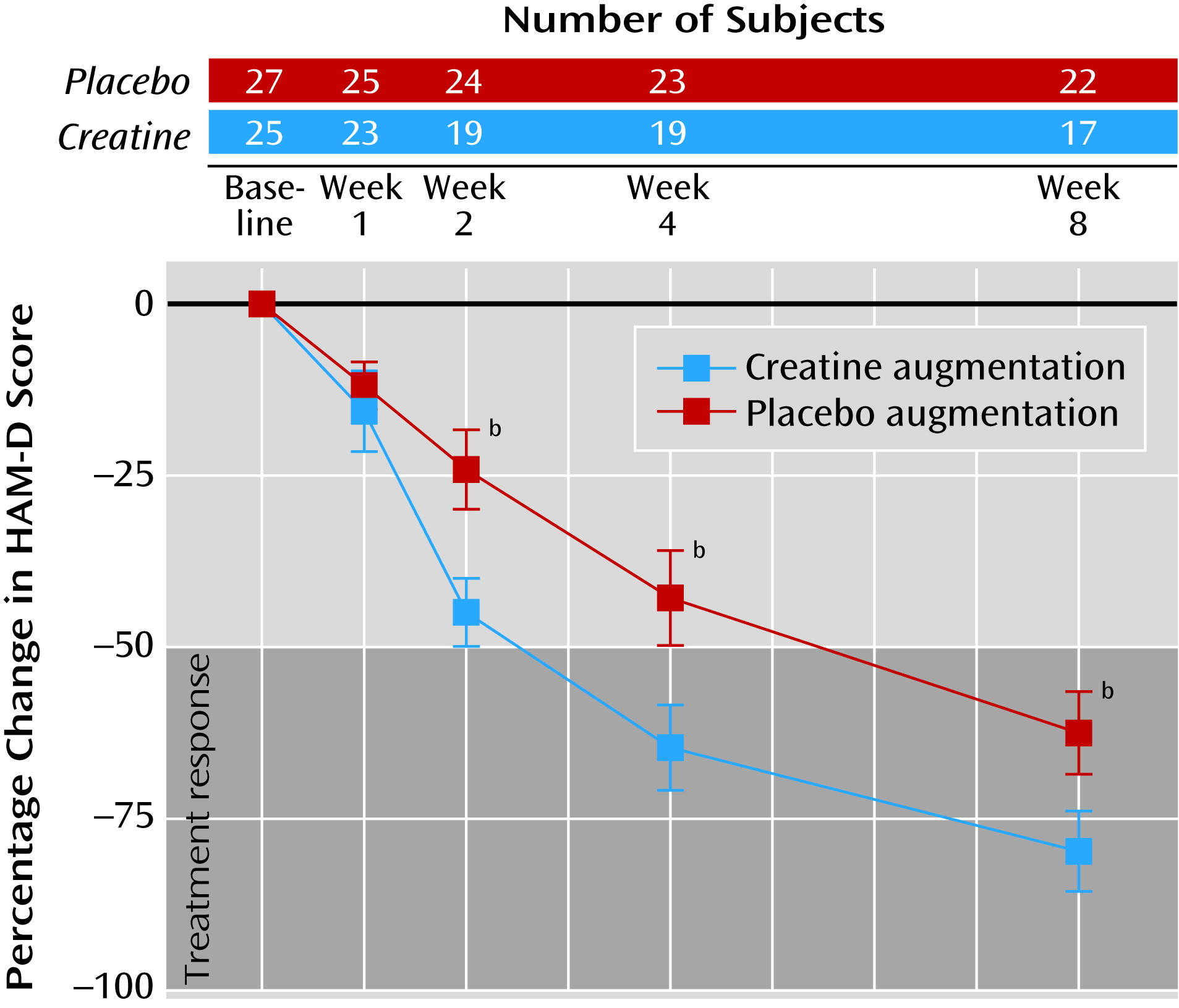
In relation to the patients who received placebo augmentation, those who received creatine augmentation showed greater improvement of depressive symptoms as early as week 2. This differential improvement between treatment groups was maintained until the end of treatment. Percentage changes and individual trajectories of HAM-D scores are presented in
Figure 2 and online supplemental Figure SF1, respectively.
For the HAM-D scores, significant interactions of visit and treatment group were observed at week 2, week 4, and week 8 (
Table 2 and
Figure 2).
Secondary Outcome Measures
Similarly, changes in MADRS scores showed significant differences in symptom improvement between treatment groups at week 2 (z=–4.87, p<0.001), week 4 (z=–4.73, p<0.001), and week 8 (z=–3.55, p<0.001). Significant improvements from baseline with creatine relative to placebo augmentation were also observed in the CGI severity subscale at week 2 (z=–2.84, p=0.005), week 4 (z=–4.24, p<0.001), and week 8 (z=–3.89, p<0.001).
The treatment response rates based on the HAM-D score in the creatine group (N=25) were 0% (N=0), 32.0% (N=8), 68.0% (N=17), and 64.0% (N=16) at weeks 1, 2, 4, and 8, respectively, while the placebo group (N=27) showed treatment response rates of 0% (N=0), 3.7% (N=1), 29.6% (N=8), and 63.0% (N=17) at weeks 1, 2, 4, and 8, respectively, as shown in online supplemental Figure SF2. There were significant effects of treatment group, favoring creatine augmentation, on the change in treatment response rate at week 2 (odds ratio=16.05, SE=18.11, p=0.02) and week 4 (odds ratio=16.54, SE=14.61, p=0.001) but not at week 8 (odds ratio=5.01, SE=5.83, p=0.17).
According to the HAM-D score, 13 (52.0%) of the patients with creatine augmentation achieved remission at week 8, while remission was achieved in seven (25.9%) of the patients in the placebo group. The remission rate at end point was higher in the creatine group than in the placebo group (odds ratio=6.92, SE=5.07, p=0.008) (online supplemental Figure SF2).
The higher response and remission rates in the creatine group remained significant in sensitivity analyses after imputation of the results from individuals who had stopped taking the study medication at week 4 for any reason (N=10), who were considered nonresponders and nonremitters.
Sensitivity analyses for the responders showed earlier improvement in the HAM-D score; odds ratios for the creatine group relative to the placebo group were significant at week 2 (odds ratio=11.68, SE=13.04, p=0.03) and week 4 (odds ratio=4.99, SE=3.04, p=0.008). Sensitivity analysis for remission (at end point) also showed a higher remission rate in the creatine group (odds ratio=3.62, SE=2.25, p=0.04).
Safety and Tolerability
All reported adverse events by week are presented in
Table 3. The overall frequencies of adverse events in the two treatment groups were similar: 43.4% (36 events) and 46.4% (45 events) of the person-examinations for creatine and placebo, respectively (χ
2=0.16, df=1, p=0.69). The most common adverse events in both treatment groups were tension headache, nausea and/or vomiting, and sleep difficulties. Most of the adverse events occurred at an early phase of treatment and improved without specific interventions. No serious adverse events were observed during the treatment period in either group. Two patients with creatine augmentation (8.0%) and one with placebo (3.7%) discontinued use of study medications at week 2 because of adverse events. The symptom clusters of nausea, tension headache, restlessness, and insomnia, which may be related to the use of SSRIs (
28), were the major reasons for intolerance of the study medications.
There were no differences in laboratory measures or BMI during the treatment period between groups (see online supplemental Table ST1). Two patients in the creatine group and one in the placebo group showed slight increases in the levels of liver transaminases beyond the upper normal limit at week 8.
As expected, mild elevation of the serum creatinine level was observed exclusively in patients receiving creatine augmentation (z=2.30, p=0.03), although there was no individual whose serum creatinine level increased beyond the normal range (online supplemental Table ST1).
Discussion
The current study is, to our knowledge, the first randomized, double-blind placebo-controlled trial to demonstrate more rapid antidepressant efficacy of the SSRI escitalopram when augmented with creatine for women with major depressive disorder.
This proof-of-concept study suggests that depression symptoms, as measured by three different scales (HAM-D, MADRS, and CGI severity subscale), may improve to a greater extent in escitalopram-treated depressed patients receiving creatine than in those receiving placebo as early as 2 weeks after the initiation of treatment. It is also noteworthy that more patients with creatine augmentation achieved early treatment response, defined a priori, during the study period.
In addition to the more rapid onset of antidepressant efficacy, a significantly higher remission rate was observed in the creatine augmentation group. The remission rate (25.9%) in our placebo group was comparable with the result (28%) from a previous large-scale study evaluating clinical outcomes of citalopram treatment for depression by the same measurement we used (
6), but the remission rate was substantially increased when creatine was added, to 52.0% (13 of the 25 patients in the creatine group), which is twice the rate observed for the group receiving the SSRI plus placebo.
Although the exact intracellular mechanism underlying adjunctive creatine’s bolstering of SSRI antidepressant efficacy remains unclear, the involvement of creatine in enhancing the phosphocreatine energy pool, thereby improving cellular bioenergetics (
10,
11), is a possible mechanism. A series of
31P-MRS studies have shown that abnormal brain bioenergetics related to major depressive disorder are likely to improve with successful treatment (
12–
16). It is intriguing that a higher baseline phosphocreatine level has been suggested as a biological marker for a better treatment response (
13).
S-Adenosyl-
l-methionine, which is a methyl donor in the production of creatine (
10) and exerts robust antidepressant efficacy, particularly in SSRI-resistant major depression (
30), may be similar to creatine in terms of increasing phosphocreatine levels in the brain (
31). A recent preliminary trial also showed that creatine augmentation improves depressive symptoms in adolescents with SSRI-resistant major depressive disorder by increasing brain phosphocreatine levels (
32). Emerging evidence suggests that in addition to modulating cellular energy metabolism, creatine exerts neuroprotective effects through mechanisms involving antiaptotic and antioxidant effects on mitochondrial functioning (
11). Future
31P-MRS studies in depressed patients treated with creatine would provide important information regarding the neurobiological basis for its effects on brain bioenergetics.
Creatine augmentation was relatively well tolerated in this study. Aside from one case report of idiosyncratic renal effects with a large dose (20 g/day) for a period of 4 weeks (
33), creatine supplementation has not been associated with major health risks (
34). Mild adverse effects, including increased body mass, muscle cramps, diarrhea, and gastrointestinal pain, have been reported in athletes who consumed 10–20 g/day of creatine (
34). Although a significant increase in serum creatinine levels from baseline was observed in patients in our creatine group, as in other previous trials (
35,
36), serum creatinine and blood urea nitrogen levels, both of which represent overall renal function, were all within normal limits during the study. According to the profile of creatine-related side effects (
34), the most frequently observed adverse events in both treatment groups, such as tension headache, nausea/vomiting, and sleep difficulties, seemed to be more directly related to the SSRI rather than to creatine (
28).
Although the difference was not significant, the discontinuation rate was higher in the creatine group (N=8, 32.0%) than in the placebo group (N=5, 18.5%). Six patients receiving creatine left the trial within 2 weeks, while three patients with placebo augmentation dropped out during the same time. The tendency toward a higher discontinuation rate with creatine augmentation, particularly during the early treatment period, may be attributed to intolerance, lack of efficacy, or both.
Although we did not find an overall higher rate of creatine-specific side effects and there appear to be no proven pharmacokinetic drug interactions with creatine (
7), intolerance of the study medication could increase if there are interactions between creatine and escitalopram. Further pharmacokinetic and pharmacodynamic studies may be necessary to examine whether creatine augmentation could alter the metabolism of SSRIs.
To consider inefficacy, we recalculated the odds ratios for remission under the assumption that the patients who dropped out prematurely did not achieve remission. The remission rate based on the HAM-D scores was still higher in the creatine group.
Our study has several limitations that merit consideration in interpreting the results. First, the study has the limit imposed by the small number of subjects to detect a relatively modest effect size from the difference between treatment groups. In addition, patient characteristics in this study were relatively homogenous, since the study included only women and had a large percentage of patients who had never taken psychotropic medications, since most were having their first major depressive episode. Although the homogeneity of this study group may have advantages in terms of statistical power, particularly in a proof-of-concept study, it limits the generalizability of the results to all patients with major depressive disorder. Third, a small portion of the current subjects, particularly those experiencing the first major depressive episode, may eventually convert to bipolar disorder even though they had no previous manic nor hypomanic episodes. Because of pilot data reporting the occurrence of a manic or hypomanic switch in patients with bipolar depression who were taking creatine (
37), future studies ensuring the long-term safety of creatine are necessary. Fourth, the study included only women with major depressive disorder because of preclinical evidence of antidepressant-like effects of creatine in female rodents (
17). Consequently, our study could not test whether the antidepressant effects of creatine are specific to women. Future studies that also include men are warranted. Fifth, given the ethnicity-based differences in the pharmacodynamics and pharmacokinetics of psychotropic medications (
38,
39), the current results from an ethnically homogenous study group need replication in a larger pool with different ethnic groups.
The present results suggest that creatine, used to augment treatment with the SSRI escitalopram, provides a promising therapeutic approach for major depressive disorder in terms of its superior efficacy, relatively good tolerability, minimal side effects, and easy attainability. Replication of the current findings is required in larger study groups and with a longer period of observation. Furthermore, on the basis of previous findings indicating that creatine supplementation increases cerebral creatine levels (
9,
32) and that changes in cerebral phosphocreatine levels may be related to antidepressant efficacy (
13,
40), future studies are required in order to assess the possibility of the cerebral phosphocreatine level as a relevant biological marker for predicting creatine-induced antidepressant efficacy.