Glycogen synthase kinase 3β (GSK-3β) is highly expressed in the brain (
1), plays a crucial role in modulating signal transduction from the extracellular space to the nucleus, and is implicated in a series of neurodevelopmental processes (
2). These diverse effects are also likely mediated by its role in phosphorylation of β-catenin, which regulates gene expression (
3). Schizophrenia is a neurodevelopmental disorder (
4) whose risk is mainly explained by genetic variation (
5) and is characterized by dysfunction in the prefrontal cortex, especially during working memory and attention (
6). Previous studies have demonstrated genetic association of
GSK-3β with diagnosis of schizophrenia (
7,
8). Other investigations have demonstrated reduced levels of GSK-3β (
9) and of its phosphorylation (
10,
11) in postmortem prefrontal cortex samples from individuals with schizophrenia. Furthermore, several molecular pathways already implicated in schizophrenia, including dopamine, serotonin, DISC1, neuregulin, and Wnt, converge onto GSK-3β (
12–
16). In particular, previous evidence robustly suggests that a dopamine D
2-signaling pathway including the serine/threonine protein kinase AKT1 modulates GSK-3β activity and its regulatory output on β-catenin (
3,
16,
17). Consistently, antipsychotic drugs blocking D
2 receptors also modulate GSK-3β expression and activity (
18).
Studies of genetic variation within
GSK-3β have also been conducted. In vitro experiments have suggested association of
GSK-3β single-nucleotide polymorphisms (SNPs) with
GSK-3β promoter activity (
8), transcriptional strength, and splicing (
19). Other studies have indicated that SNPs in
GSK-3β affect temporal lobe volume in individuals with major depression (
20) and schizophrenia (
21). Furthermore, association of
GSK-3β variants with clinical measures related to mood disorders (
22,
23) and with susceptibility to schizophrenia (
8) has been reported. However, to our knowledge, no previous study has evaluated the functional molecular effects of
GSK-3β polymorphisms in humans together with their direct implications for brain physiology relevant to schizophrenia.
The aim of this study was to explore molecular correlates of GSK-3β genetic variation as well as its relevance to several phenotypes related to schizophrenia. Since genetic variation does not directly cause behavioral phenotypes but rather has molecular effects that influence neural systems-level processing, we investigated the possible effect of GSK-3β polymorphisms on a series of progressively more complex phenotypes potentially linked to risk for schizophrenia. Specifically, after conducting computer predictions of functional SNPs, we tested the association of genetic variation (rs12630592) with GSK-3β mRNA levels in postmortem prefrontal cortex, which was further validated in terms of protein expression and phosphorylation in peripheral lymphocytes in another independent sample. Demonstration of association of this SNP with prefrontal gene expression prompted the question of whether this molecular mechanism underlies more complex phenotypes (i.e., prefrontal activity during cognitive performance and prefrontal cortical thickness in healthy humans). Robust effects of rs12630592 on these phenotypes further suggested investigation of this SNP regarding its relationship with the diagnosis of schizophrenia, the most complex of the phenotypes examined.
Method
Bioinformatic Modeling of Molecular Effects of GSK-3β Variants
We used bioinformatic tools (rVISTA 2.0, SpliceAid 2, NNSPLICE, rBLOSUM64, SIFT, PolyPhen, and miRBase) to predict
GSK-3β SNPs with potential effects on molecular regulatory mechanisms possibly affecting
GSK-3β expression (see the
data supplement that accompanies the online edition of this article). Based on these bioinformatic analyses, we further investigated variants associated with predicted functional effects. In order to focus our analysis on genetic variation with possible common biological relevance in humans, we selected SNPs with a known minor allele frequency >0.03, which allows investigation of a large enough number of individuals with the minor allele to detect genotype effects in postmortem and in vivo studies (
24). Thus, a publicly available database of postmortem brains was interrogated to study association of the remaining SNPs with
GSK-3β mRNA expression in the prefrontal cortex.
Association of GSK-3β Variation With Postmortem Prefrontal Expression of GSK-3β mRNA
Data from 215 brains of nonpsychiatric individuals (males, N=152; mean age, 33.1 years [SD=21.0]; brain pH=6.5 [SD=0.3]; postmortem interval, 30.6 hours [SD=15.4]), with available information on age, sex, race, postmortem interval, pH, and smoking status, were analyzed. These data were obtained from the largest postmortem collection publicly available (
http://braincloud.jhmi.edu [
25]) (see the online
data supplement). We focused on association of prefrontal mRNA expression with
GSK-3β SNPs that had been predicted by bioinformatic tools and that had a minor allele frequency >0.03. Based on these selection criteria, data related to two SNPs (rs12630592 and rs6797689) were available from the postmortem collection.
For GSK-3β rs12630592, the postmortem sample included Caucasian and African American subjects with GG (N=44), GT (N=83), and TT (N=88) genotypes. For GSK-3β rs6797689, the sample included subjects with CC (N=170), CT (N=43), and TT genotypes (N=2). For both SNPs, genotype groups did not differ in terms of age, sex, smoking status, postmortem interval, and pH, even after collapsing the GSK-3β rs6797689 TT and CT groups into a single T carrier group (all p values >0.05).
Analysis of variance (ANOVA) was used to explore association of rs12630592 and rs6797689 with prefrontal mRNA expression. Given that rs6797689 did not demonstrate any statistically significant association with prefrontal mRNA expression, we focused further in vivo analyses on rs12630592.
Healthy Human Subjects
DNA was extracted from whole blood from a sample of 424 healthy humans (see the online
data supplement) using standard procedures.
GSK-3β rs12630592 was determined by real-time polymerase chain reaction (see the
data supplement). Participants underwent one or more of the procedures described below.
Association of rs12630592 With GSK-3β and β-Catenin Expression in Peripheral Blood Mononuclear Cells (PBMCs)
Blood samples were drawn from 31 healthy individuals (female, N=17; mean age, 27.5 years [SD=5.9]; GG genotype, N=9; GT genotype, N=10; TT genotype, N=12). PBMCs were isolated (
26), and aliquots (2 μl) of samples were used for the protein determination (see the
data supplement). Immunoblot was performed using selective antibodies against GSK-3β, P-Ser9-GSK-3β, β-catenin, and α-tubulin (see the
data supplement). Optical density values of GSK-3β and β-catenin were normalized to α-tubulin for variation in loading and transfer. The levels of P-Ser9-GSK-3β were normalized to total GSK-3β. ANOVA was used for statistical analyses of averaged and normalized protein values.
Association of rs12630592 With Prefrontal Physiology During Cognition
A total of 209 healthy subjects underwent functional MRI (fMRI) during cognitive performance. Of these, 108 individuals (female, N=50; mean age, 29.8 years [SD=7.2]; IQ=110.1 [SD=12.5]) performed the one-, two-, and three-back versions of the
n-back working memory task (
27) (GG genotype, N=32; GT genotype, N=59; TT genotype, N=17). We used a three-run block design in which each block consisted of eight alternating zero- and one-, two-, or three-back tasks. Presentation of the runs was counterbalanced. Genotype groups were matched for age, IQ (WAIS), and handedness (Edinburgh Handedness Inventory [
28]) (all p values >0.4) but not for sex (χ
2=14.3, p<0.01).
A total of 151 subjects (female, N=85; mean age, 26.3 years [SD=5.6]; IQ=110.7 [12.0]) performed the variable attentional control task, eliciting parametric increase of attentional control (
29) (GG genotype, N=46; GT genotype, N=86; TT genotype, N=19). The variable attentional control task is an event-related design (
17,
29–
31) that allows for investigation of brain activity during three levels of attentional control (low, intermediate, and high), which are obtained by manipulating both the relative directions of arrows with different sizes and the related cue words (see the
data supplement).
Genotype groups were matched for age, sex, IQ, and handedness (all p values >0.1). Fifty-two individuals performed both tasks used in this study.
fMRI Data Acquisition and Analysis
fMRI data were acquired using a 3-T General Electric scanner (General Electric, Fairfield, Conn.). The analysis was completed using Statistical Parametric Mapping 8.0 (
http://www.fil.ion.ucl.ac.uk/spm). After preprocessing (see the
data supplement), the individual contrast images relative to the
n-back (one-, two-, and three-back tasks compared with zero-back tasks) and variable attentional control (low, intermediate, and high levels of attentional control compared with baseline) tasks were used in random-effects models for group-level analysis. In particular, ANOVAs were performed on these contrasts, with genotype as the predictor and working memory or attentional control load as repeated-measures factors. Sex was used as a covariate of no interest in the
n-back ANOVA to account for nonhomogeneous distribution of this variable. We used a statistical threshold with a p value <0.001 (minimum cluster size [k]=5), with further family-wise error small-volume correction at a p value <0.05, using as volume of interest the Wake Forest University PickAtlas (
http://fmri.wfubmc.edu/cms/software#PickAtlas) Brodmann’s areas (BAs), in which significant clusters were located (i.e., BA 10 for the variable attentional control task and BA 46 for the
n-back task). These regions of interest were chosen a priori based on our investigation of prefrontal phenotypes and on the well-established association between these tasks and activity in the dorsolateral prefrontal cortex (BA 9/10/46) (
27,
30,
32). Fisher’s post hoc test was performed on blood-oxygen-level-dependent (BOLD) responses extracted from significant clusters using MarsBar (
http://marsbar.sourceforge.net/) (see the
data supplement).
Pearson’s test was used to investigate brain activity-behavior correlations. In particular, we reasoned that the repeated-measures design measuring brain activity and behavior at different cognitive loads within a single cognitive domain allows for maximization and better characterization of the relationship between brain and behavior if differences between the different cognitive loads are considered. Therefore, we correlated the delta of BOLD responses between loads with the delta of percent-correct responses between loads as follows: n-back task: three-back minus two-back activity compared with two-back minus three-back accuracy (a similar procedure was used for deltas between two-back and one-back tasks); variable attentional control task: high- minus intermediate-loads activity compared with intermediate- minus high-loads accuracy (the same method was used for deltas between intermediate and low loads).
ANOVA was used for analysis of behavioral data.
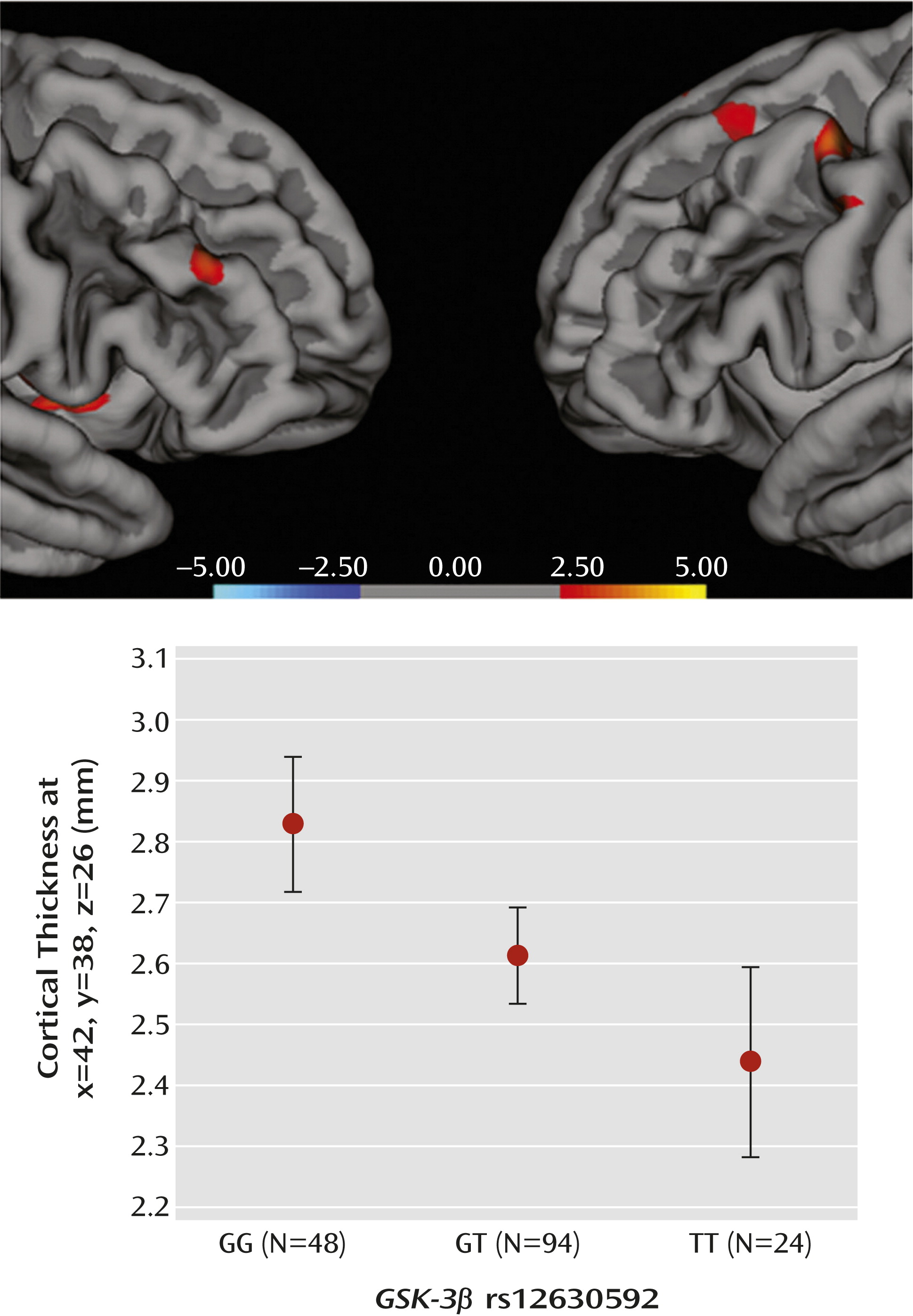
Association of rs12630592 With Prefrontal Cortical Thickness
A total of 166 healthy subjects (female, N=103; mean age, 26.11 years [SD=6.69]; IQ=107.43 [SD=12.66) underwent structural MRI (GG genotype, N=48; GT genotype, N=94; TT genotype, N=24). Genotype groups were matched for age, sex, IQ, and handedness (all p values >0.5). After acquisition of three-dimensional images (see the online
data supplement), MRI-based quantification of cortical thickness was performed using Freesurfer 5.0.0 (
http://surfer.nmr.mgh.harvard.edu/) (see the
data supplement). For each hemisphere, differences in prefrontal cortical thickness between genotype groups were tested by computing a general linear model of the effects of genotype (GG compared with TT, GG compared with GT, and GT compared TT) on prefrontal cortical thickness at each vertex. Age and sex were used as covariates of no interest. A statistical threshold was set at a p value <0.001. To control for the effects of multiple testing, permutation analysis (10
5 permutations) was performed on the mean cortical thickness values extracted from the prefrontal clusters associated with a main effect of genotype using the Resampling software program (
http://core.ecu.edu/psyc/wuenschk/StatHelp/Resampling.htm), which adopts a classical “randomization without replacement” algorithm.
Association of rs12630592 With Diagnosis of Schizophrenia
A case-control study was performed in a sample of Caucasian individuals (N=1,057), including 459 patients with schizophrenia (female, N=116; mean age, 36.65 years [SD=11.11]) and 598 healthy subjects (female, N=313; mean age, 26.86 years [SD=7.53]) recruited from the Apulia region. The Structured Clinical Interview for DSM-IV Axis I Disorders (
33) was used to diagnose schizophrenia and to exclude any psychiatric disorders in comparison subjects. Personal and family histories of psychiatric disorders were ruled out for all comparison subjects. Genotype groups deviated from Hardy-Weinberg equilibrium in both the patient and comparison groups (p<0.01). A quality-control check was also performed (see the
data supplement). Logistic regression was performed on
GSK-3β rs12630592 genotype data using HAPSTAT (
http://www.bios.unc.edu/~lin/hapstat/). Results were corrected for multiple comparisons considering the number of models applied (i.e., dominant, recessive, and additive).
The experimental protocol was approved by the local institutional review boards, and participants provided written informed consent after receiving complete description of the study.
Results
Bioinformatic Modeling of Molecular Effects of GSK-3β Variants
Computer predictions indicated that 50 SNPs within GSK-3β have molecular effects on gene function (see the online
data supplement). Eight of these SNPs had a known minor allele frequency >0.03 and thus were considered and investigated in the subsequent step of our study. Among these eight SNPs, data related to rs12630592 and rs6797689 were available for postmortem biological validation of the predictions and therefore were of particular interest in our hierarchical strategy. In particular, the NNSPLICE prediction algorithm and SpliceAid2 database indicated that the intronic variant rs12630592 creates a strong 3′-splice site (score=0.95 on a scale from 0 to 1) and a binding site for the SRp20 exonic splicing enhancer. Furthermore, rs12630592 destroys a binding site for MBNL1, a splicing regulatory factor that can act as a silencer or enhancer, depending on the context. These effects may modify splicing patterns with possible regulatory outcomes on the amount of the total
GSK-3β transcript because of mechanisms of mRNA surveillance.
Computer analysis of rs6797689 predicted that this deep intronic variation destroys a binding site for the exonic splicing enhancer SRp30c and creates a binding site for the exonic splicing silencers hnRNP A1 and hnRNP A2/B1. Generally, these events do not imply splicing alterations.
Association of GSK-3β Variation With Postmortem Prefrontal Expression of GSK-3β mRNA
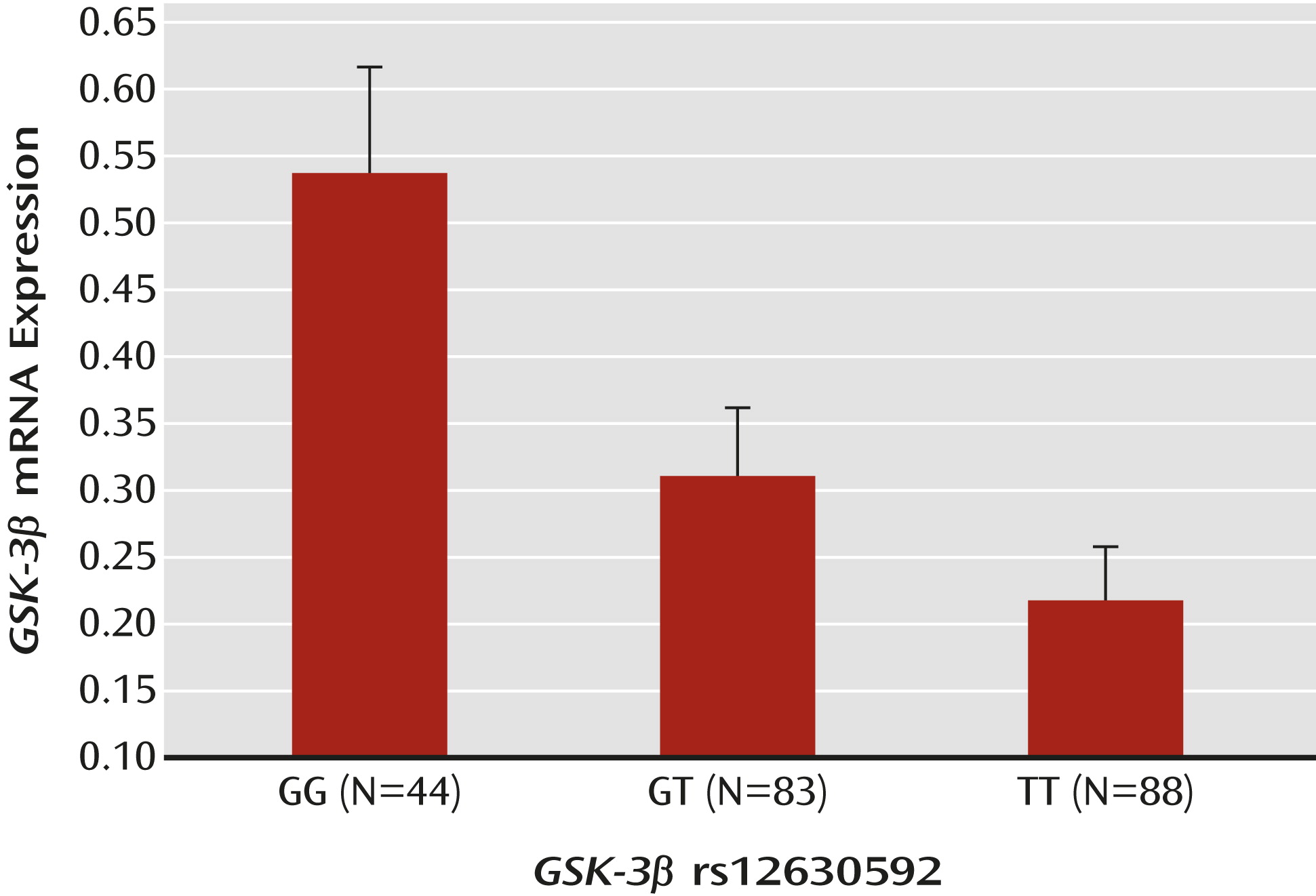
Postmortem data were available for two of the eight selected SNPs, rs12630592 and rs6797689. ANOVA indicated a main effect of rs12630592 on GSK-3β mRNA expression (F=7.18, df=2, 222, p=0.0009). Fisher’s post hoc analysis revealed reduced mRNA expression in subjects with the TT genotype compared with those with the GG genotype (p<0.0002) but no statistical difference between subjects with the TT genotype and those with the GT genotype. Furthermore, GG subjects exhibited greater mRNA expression than GT subjects (p=0.008) (
Figure 1). There was no race effect on the association between genotype and GSK-3β mRNA expression (see the
data supplement).
There was no effect of rs6797689 genotype on GSK-3β mRNA expression, even after combining T carriers into one group. Based on these results, we restricted all subsequent in vivo analyses to rs12630592.
Association of rs12630592 With GSK-3β and β-Catenin Expression in PBMCs
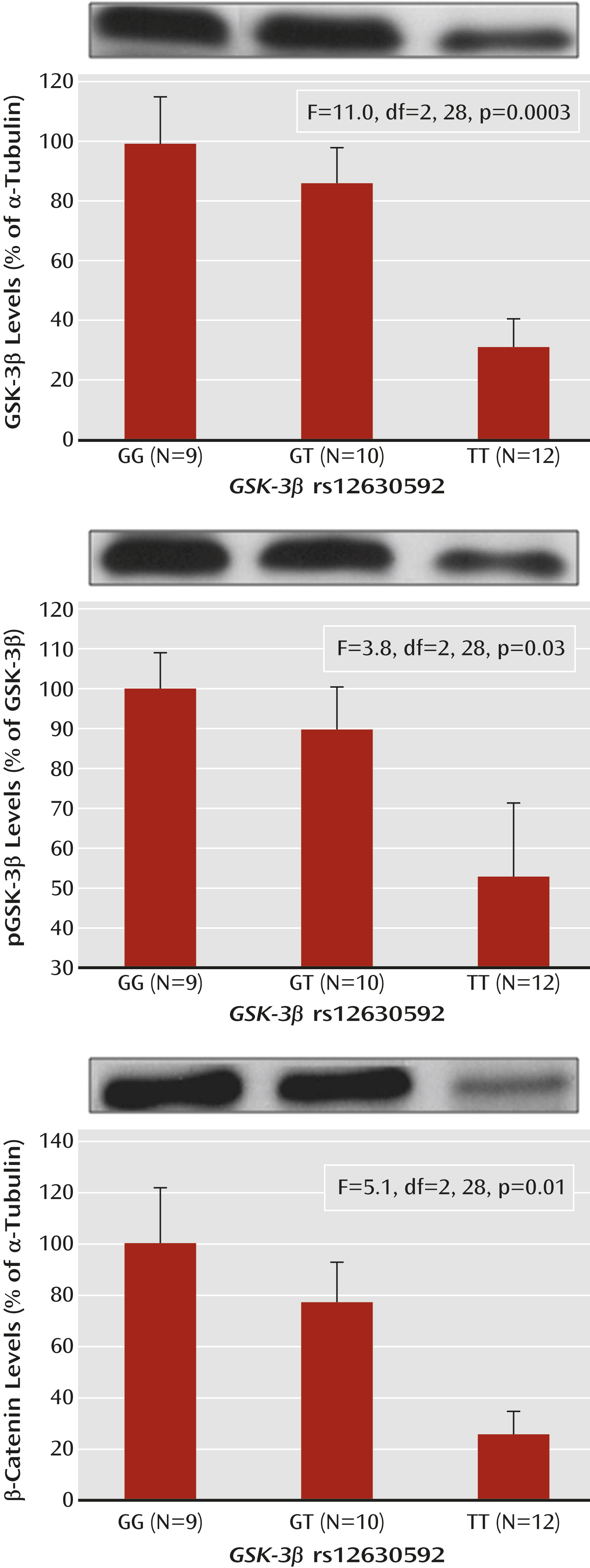
ANOVA indicated a main effect of rs12630592 on GSK-3β protein expression (F=11.0, df=2, 28, p=0.0003) and phosphorylation of the Ser9 residue (F=3.8, df=2, 28, p=0.03) in PBMCs. Fisher’s post hoc test revealed that TT subjects have attenuated immunoreactivity of GSK-3β protein and of its phosphorylation compared with GG subjects (expression, p=0.0001; phosphorylation, p=0.01) and GT subjects (expression, p=0.002; phosphorylation, p=0.04) (
Figure 2). Furthermore, there was a main effect of rs12630592 on β-catenin protein expression (F=5.1, df=2, 28, p=0.01), with TT subjects having reduced measures compared with GG (p=0.004) and GT (p=0.03) subjects (
Figure 2). No statistical difference was found between GG and GT subjects in these analyses.
Association of rs12630592 With Prefrontal Physiology During Cognition
n-Back task.
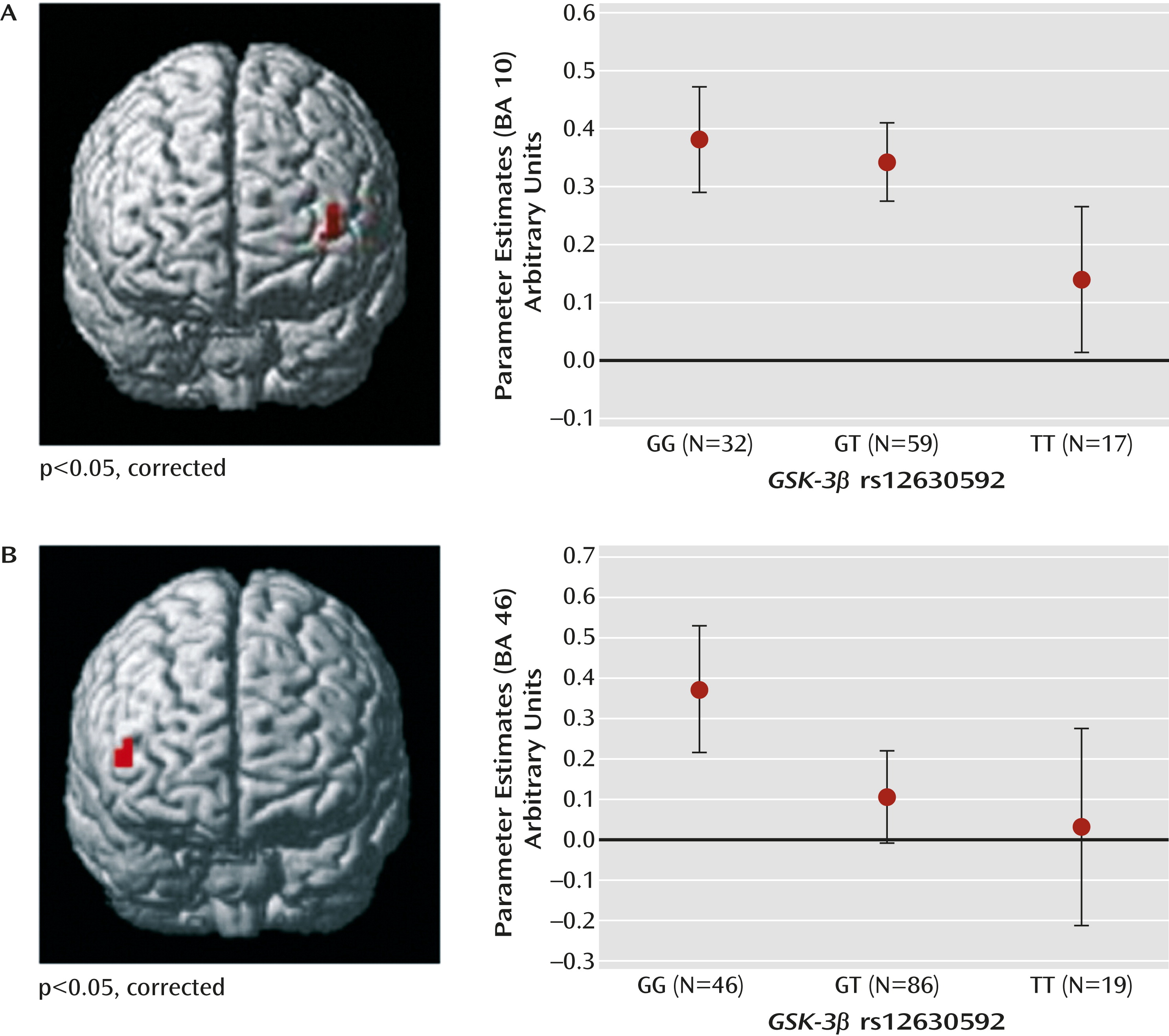
No main effect of genotype or genotype-by-load interaction was present in behavioral data for this sample (all p values >0.2). Thus, the effect of the rs12630592 genotype on brain responses during working memory processing in this sample reflects how the brain processed working memory and not how individuals scored on the test. Statistical Parametric Mapping ANOVA of imaging data indicated a main effect of rs12630592 on a cluster in the left dorsolateral prefrontal cortex (Montreal Neurological Institute [MNI] coordinates: x=–40, y=56, z=6; BA 10; k=7; family-wise error-corrected, p=0.04) (
Figure 3). Analysis of parameter estimates extracted from this cluster revealed that TT subjects have attenuated dorsolateral prefrontal cortex activity compared with GT (p=0.005) and GG (p=0.002) subjects. No significant difference was present between GG and GT subjects (
Figure 3). No genotype-by-load interaction was found on brain activity.
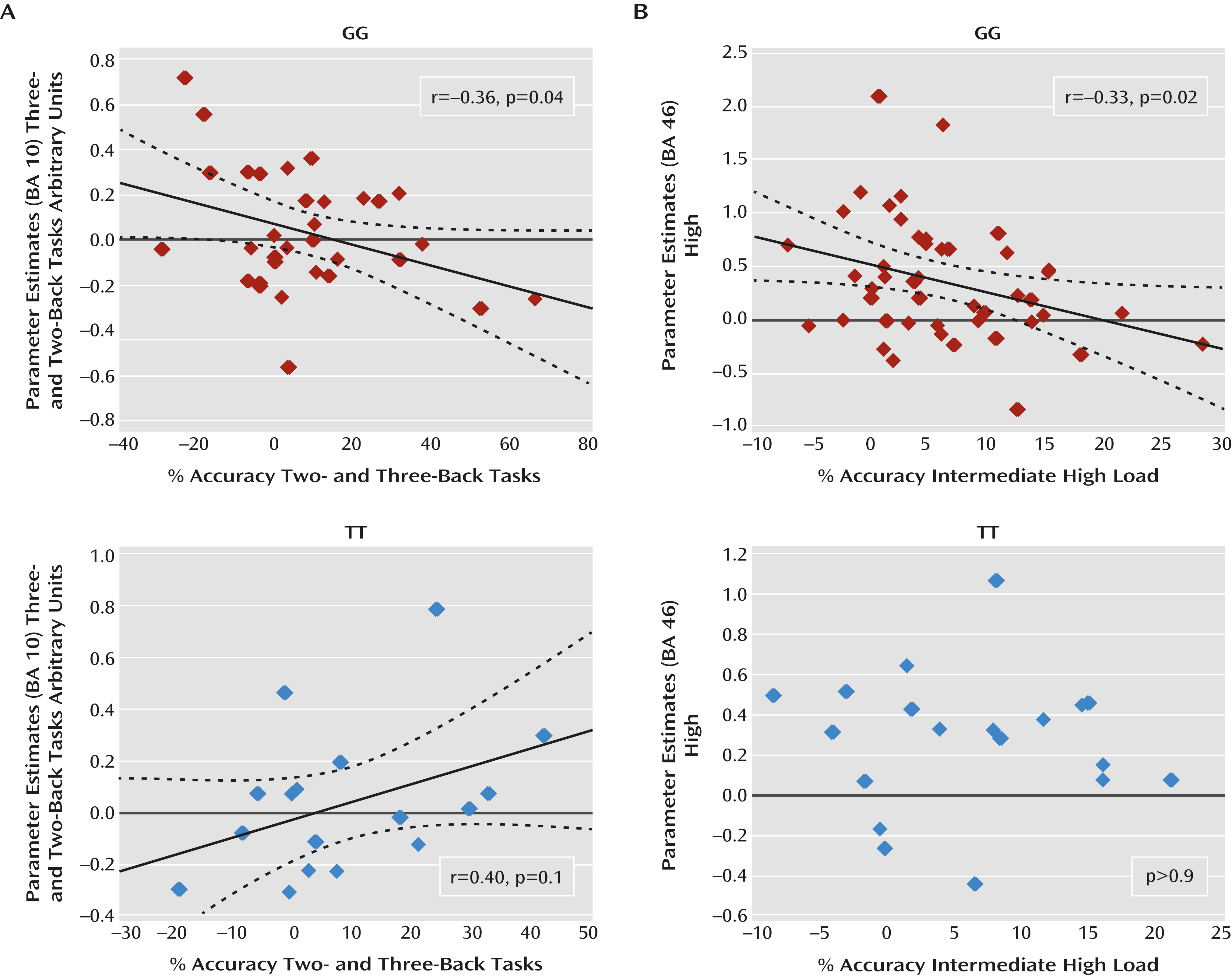
Pearson’s test also indicated a relationship between activity in the cluster showing a main effect of genotype and indices of behavioral accuracy. In particular, in GG subjects, there was a significant negative correlation between the difference in prefrontal BOLD responses (three-back minus two-back task) and the difference in accuracy (two-back minus three-back task) at high cognitive loads (r=–0.36; p=0.04). In contrast, a positive correlation was present in TT subjects (r=0.40; p=0.1) (
Figure 4). A difference test indicated that the slope of these correlations was statistically different (p=0.01). Results for GT subjects did not demonstrate a significant correlation. No significant correlations were present when investigating deltas between two-back and one-back task data.
Variable attentional control task.
Again, no main effect of genotype or genotype-by-load interaction was present in behavioral fMRI data. Imaging data again revealed a main effect of rs12630592 on a region in the right dorsolateral prefrontal cortex similar to that found in the
n-back task analysis (MNI coordinates: x=50, y=45, z=7; BA=46; k=5; corrected p=0.04) (
Figure 3). Parameter estimates extracted from this cluster indicated that TT and GT subjects had attenuated responses compared with GG subjects (p=0.02 and p=0.007, respectively). No significant difference was present between TT and GT subjects (
Figure 3).
Consistent with the
n-back task results, Pearson’s test revealed in GG subjects a significant negative correlation between the difference in prefrontal BOLD response (high minus intermediate) and the difference in accuracy (intermediate minus high) at high cognitive loads (r=–0.33; p=0.02) (
Figure 4). No significant correlation between these variables was present in TT or GT subjects. Furthermore, no significant correlation was found when analyzing deltas between intermediate and low levels of attentional control processing.
Association of rs12630592 With Prefrontal Cortical Thickness
ANOVA indicated a main effect of rs12630592 on cortical thickness in prefrontal clusters, with greater values in GG relative to GT and TT subjects, as well as in GT relative to TT subjects (
Figure 5, also see Tables S1 and S2 in the online
data supplement). No significant effects were present in the inverse comparisons.
Association of rs12630592 With Diagnosis of Schizophrenia
Logistic regression revealed association of the rs12630592 T allele with diagnosis of schizophrenia under a recessive model (p=0.002). The effect size of this association was an odds ratio of 1.13, with a 95% confidence interval of 0.95–1.34 (minor allele T frequency: patients=0.45; comparison subjects=0.41). These results were still significant after correction for the number of models tested (p=0.006). Dominant and additive models were not statistically significant.
Discussion
We used a translational genetics approach to investigate the relevance of
GSK-3β to aspects of human brain function and behavior related to schizophrenia. The first level of this strategy entailed computer predictions. Results indicated that the intronic SNP rs12630592 may regulate splicing, possibly triggering mechanisms of mRNA surveillance, thus affecting the abundance of the total transcript (
34,
35). In fact, the second level of this strategy addressed the functional relevance of this SNP to the abundance of the transcript in postmortem human prefrontal cortex. Consistent with the bioinformatic prediction, nonpsychiatric TT subjects exhibited reduced GSK-3β mRNA in the prefrontal cortex compared with GG subjects, as measured with a probe positioned in the gene to detect the main transcript variant. In line with the postmortem results, in vivo findings revealed reduced GSK-3β protein expression and reduced Ser-9 phosphorylation in PBMCs from healthy TT subjects. Interestingly, similar reductions in GSK-3β mRNA, protein expression, and its phosphorylation have also been observed in lymphocytes and postmortem prefrontal cortex of individuals with schizophrenia (
9–
11). A crucial downstream effect of the active Ser-9 dephosphorylated form of GSK-3β is the degradation and thus inactivation of β-catenin (
3). In PBMCs, we found association of the TT genotype with reduced phosphorylation of GSK-3β and with reduced protein expression of β-catenin. Regardless of the specific mechanism, the more parsimonious interpretation of these data is that rs12630592 is associated with consistent effects on GSK-3β expression and phosphorylation, which also affect downstream signaling of gene expression with possible effect on the neuronal function of relevance to schizophrenia (
11) and antipsychotic treatment (
18).
We next validated these molecular effects on more complex phenotypes. Earlier studies have demonstrated that selective deletion of
GSK-3β in neural progenitors results in overexpansion of the neural progenitor cells and thinner cortex (
36). Our results demonstrate that the T allele predicts reduced prefrontal cortical thickness in healthy humans, suggesting that genetic variation in GSK-3β may confer risk for altered dynamics of neurodevelopment (18). Furthermore, the T allele of rs12630592 was associated with reduced prefrontal activity during working memory and attentional control, regardless of cognitive load. Additionally, rs12630592 genotype differentially predicted the load-dependent relationship between brain activity and cognitive behavior. At greater working memory and attentional control demands, greater load-mediated increase in prefrontal activity predicted lower decrease in behavioral accuracy in GG subjects. In other words, greater dorsolateral prefrontal cortex activity in GG subjects appeared to reflect greater behavioral accuracy at high cognitive loads. This relationship was not present in GT subjects, while in TT subjects, a nonsignificant association in the opposite direction was observed. An interpretation of the main effect of genotype and of the correlations with behavior is that greater dorsolateral prefrontal cortex activity in GG subjects is functional to better behavioral performance at high cognitive loads, while this is not the case in individuals carrying at least one T allele. More generally, these data suggest that rs12630592 is associated with prefrontal physiology during cognition altering the load-dependent architecture of its relationship with behavior. Abnormal prefrontal response (
31,
37) during working memory and attentional processing are considered key features of schizophrenia.
In line with these results, the TT genotype was associated with schizophrenia in a recessive model, suggesting that the presence of the T allele on both chromosomes is necessary to increase risk. With an odds ratio of 1.13, our results of the association with diagnosis are consistent with earlier evidence indicating a highly polygenic model as best suited to explain risk for schizophrenia (
38), such that genetically determined individual differences across domains of brain development and function may form a diathesis for this brain disorder (
13). The association with diagnosis is also consistent with previous studies that have evaluated other
GSK-3β polymorphisms (
7,
8). Of note, SNPs associated with diagnosis of schizophrenia in these studies (i.e., rs3755557 [
8], rs7624540, rs4072520, and rs6779828 [
7]) are in high linkage disequilibrium with rs12630592 (
http://hapmap.ncbi.nlm.nih.gov/). The association of rs12630592 with diagnosis of schizophrenia would not survive the statistical criteria for genome-wide significance, and it requires further study. However, the stringent logic of our hierarchical approach and the consistency of our results from molecular to more complex in vivo phenotypes provide support for this association not being spurious.
A limitation of this study is that the multiple associations that we have demonstrated between rs12630592 and different phenotypes do not imply reciprocal biological relationships because each analysis was performed using data sets from different samples. However, the fact that these associations have been found in samples that do not completely overlap may suggest the functional relevance of this polymorphism to the biology of CNS in the general population. Another important point is that it cannot be concluded that our prefrontal findings are specific to schizophrenia because prefrontal anomalies have been associated with other brain disorders (
39,
40). However, the relationship between schizophrenia and prefrontal function is well established, such that the association that we found between rs12630592 and prefrontal phenotypes is relevant to this brain disorder but not exclusively.
Acknowledgments
The authors thank Riccarda Lomuscio, B.A., for assistance with administrative procedures, Venkata S. Mattay, M.D., for helpful discussion of the results, and Vincenzo Pierro, M.D., and Enrico D’Ambrosio, M.D., for assistance with the data analysis.