Attention deficit hyperactivity disorder (ADHD) is defined by developmentally inappropriate levels of inattention and hyperactivity-impulsivity that typically emerge during the preschool years and often persist into adulthood, causing functional disability throughout the lifespan. Longitudinal studies of inattentive and hyperactive-impulsive preschool children indicate both stability and variability of these behaviors over time (
1,
2). Relative to comparison subjects, a much greater portion of these high-risk preschool children subsequently meet criteria for ADHD. Nevertheless, approximately half of 3- and 4-year-old children identified by parent ratings of behavioral problems no longer had difficulties by 6 years of age (
3).
Several factors have been associated with variability in the trajectory of ADHD, including age at initial assessment (
4,
5), the presence of comorbid disruptive behavior disorders (
4), and objectively measured behavioral inhibition deficits (
6). Yet, these findings provide limited insight into the mechanisms that alter the course of ADHD across development. From a developmental psychopathology perspective (
7,
8), ADHD is not the result of a fixed deficit but rather a clinical manifestation of neurodevelopmental vulnerability whose trajectory is mediated by changes in brain structure and function in response to an array of interacting genetic and environmental factors throughout development.
Consistent with this view, longitudinal studies (
9,
10) indicate that children with ADHD follow a sequential pattern of cortical development similar to typically developing comparison subjects, but development is delayed by as many as 2–3 years throughout most of the cortex. Furthermore, changes in cortical thinning have been associated with clinical outcomes, such that children with ADHD with poorer outcomes had “fixed” thinning of the left medial prefrontal cortex, and children with better outcomes had right parietal normalization, suggesting compensatory cortical change (
9). Additionally, cortical thinning in the dorsolateral prefrontal cortex, the anterior cingulate, and the inferior parietal lobe that continues into adulthood has been linked to the persistence of ADHD symptoms (
11). Based on differences in cortical thickness between individuals with and without persisting symptoms, a 33-year follow-up of children with ADHD concluded that diagnostic remission may result from compensatory maturation of the brain (
12).
Consistent with these structural neuroimaging findings, preliminary functional MRI data indicate that brain activation during inhibition parallels the degree of symptom persistence in adolescents who had ADHD in childhood, with those with remitted ADHD more closely resembling comparison subjects than those with persistent ADHD (
13). Although not consistently observed (
14), neuropsychological test performance of adolescents with childhood ADHD seems to vary as a function of ADHD persistence; only those who continued to meet criteria for ADHD at follow-up differed significantly from comparison subjects on measures of working memory, inhibitory control, and sustained attention (
15–
17). However, the absence of baseline neuropsychological measures in these studies limits our ability to link changes in neuropsychological functioning to symptom trajectories. Nevertheless, better cortical and neurocognitive functioning appear to be associated with a diminution of ADHD symptoms from childhood through adulthood (
9,
11–
13,
15–
20).
Elucidating the relationships between neurocognitive development and ADHD trajectories has been hampered, however, because existing studies include only two or three assessment points, one in middle childhood and the other(s) in adolescence or early adulthood. This limits our ability to carefully examine developmental trajectories during early childhood when ADHD is first emerging and when trajectories are most variable (
1,
2). Determining relationships between early neurocognitive development and the emergence of ADHD would not only have considerable heuristic value, but could also have a substantial impact on the development of early interventions (
8,
21). A further limitation is that most studies have focused on the presence or absence of an ADHD diagnosis at follow-up. Given that ADHD symptoms present on a continuum (
22), a dimensional approach (
23) with multiple time points would provide greater sensitivity to detecting factors associated with change in symptoms and impairment.
In this longitudinal study, we employed a dimensional approach with multiple assessment points to test two hypotheses that might account for the varied trajectories among preschool children characterized by inattention and hyperactivity-impulsivity. Based on research indicating that objectively measured inhibition deficits in preschoolers predict subsequent severity of ADHD (
6), hypothesis 1 posits that poorer outcomes are associated with early neurocognitive dysfunction and that individuals with positive behavioral trajectories are phenocopies without the early neural dysfunction that underlies ADHD.
Hypothesis 2 posits that the often-observed decline in symptoms with age is accounted for by the degree to which the later development of higher cortical circuitry and function can compensate through top-down regulatory control (
18). Thus, irrespective of baseline neuropsychological functioning, individuals with greater overall neurocognitive growth over time will show a trajectory characterized by greater clinical improvement. In contrast, those with less robust cognitive growth over time will show a persisting or worsening symptom pattern over development.
The study recruited 3- and 4-year-old children who were characterized as at risk for ADHD based on elevated symptom ratings from parents or teachers. Parent and teacher ratings of ADHD symptoms and impairment were acquired every 6 months for four consecutive follow-up years; neuropsychological functioning was evaluated annually. Using hierarchical linear modeling, individual trajectories were identified based on behavioral ratings. Subsequently, we determined the degree to which neuropsychological functioning at baseline (hypothesis 1) and change in neuropsychological functioning from baseline (hypothesis 2) accounted for trajectory variability across children.
Method
Participants
One hundred thirty-eight children were recruited through screenings at preschools and referrals from preschools and local pediatric and mental health providers in the New York metropolitan area. The sample was ethnically and racially diverse (
Table 1). To be included in the study, children had to be rated by parents or teachers as having at least six separate symptoms of hyperactivity-impulsivity and/or inattention, as indicated by a rating of “often” or “very often” on the ADHD Rating Scale–IV (
24). Thus, children did not necessarily meet diagnostic criteria for ADHD, but they exhibited varying levels of symptom severity and were symptomatic in at least one setting at the time of recruitment. Children were excluded if they had a full-scale IQ less than 80 (as measured by the Wechsler Preschool and Primary Scale of Intelligence, 3rd ed.), if they had a neurological or pervasive developmental disorder, if they were taking systemic medication for a chronic medical condition (including ADHD), or if the parent or child did not speak English.
Most children had an ADHD diagnosis (N=108; 78.3%) or functional impairment at baseline according to parent (N=127; 92%) or teacher report (N=116; 84%) on the Children’s Problem Checklist (see Measures section), such that all children had impairment rated by at least one informant. No child was taking systemic medication at the time of recruitment, but the number of children receiving medication treatment increased over time (see Table S1 in the
data supplement that accompanies the online edition of this article). Thirty-four children were being treated with medication by the time they were 8 years old. Parents were asked to rate children’s behavior while not on medication, either in the evening when it had worn off or over the weekend when some children were not taking medication. Stimulant and nonstimulant ADHD medications, but not antipsychotics or antidepressants, were withheld on all assessment days.
Procedure
Parents and teachers rated children’s ADHD symptoms and impairment when they were 3 and 4 years old and then at nine semiannual follow-up visits (mean age at final follow-up, 8.81 years [SD=0.51]). Data were collected between June 2004 and May 2012. Neuropsychological functioning in children was assessed using the NEPSY at baseline and at three subsequent annual follow-up visits (mean age at last NEPSY administration, 7.35 years [SD=0.51]). The study was approved by the university’s institutional review board, and written informed consent was obtained from parents.
Measures
ADHD Rating Scale–IV (24).
This scale, based on the 18 DSM-IV symptoms, was completed by parents and teachers. Symptoms are rated on a 4-point scale ranging from 0 (never or rarely) to 3 (very often). This scale has good psychometric properties for both school-age (
24) and preschool (
25) children. The average correlation between parent and teacher reports across all time points in our sample was 0.31. Cronbach’s alpha values ranged from 0.93 to 0.96 and from 0.91 to 0.96 for teacher and parent reports, respectively.
Children’s Problem Checklist (26).
Parent and teacher reports with this psychometrically sound measure of impairment in young children were used to characterize impairment resulting from ADHD. Items are rated on a 4-point scale consisting of no, mild, moderate, or severe problems. The mean correlation between parent and teacher report was 0.17. Cronbach’s alpha values ranged from 0.74 to 0.85 and from 0.64 to 0.81 for teacher and parent reports, respectively.
NEPSY (27).
The NEPSY assesses neuropsychological functioning in five domains: attention/executive, language, sensorimotor, visuospatial, and memory. It was administered by well-trained graduate students. In the normative sample, test-retest reliability for the five domains ranged from 0.70 to 0.91 for children 3–4 years old and from 0.79 to 0.87 for children 5–12 years old (
27). In our sample, Cronbach’s alpha values for the five domains at baseline and three successive follow-up visits were 0.66, 0.74, 0.71, and 0.66, respectively. Given research suggesting a single factor underlying the test domains (
28), we used a mean of the domain scores to assess neuropsychological functioning.
Nakao-Treas Socioeconomic Prestige Index (29).
This index was used to measure socioeconomic status at baseline. High scores on this index are indicative of higher socioeconomic status. Mothers’ and fathers’ scores were separately coded, and the higher of the two was adopted to indicate family socioeconomic status.
Missing Data
The mean number of time points for data on ADHD symptoms and impairment included in the longitudinal analysis was 7.55 (SD=2.78). Of the sample, 38% (N=53) had complete data for all 10 points in the study, 33% (N=46) had seven to nine data points, 14.5% (N=20) had four to six data points, and 13.8% (N=19) had three or fewer data points. Participants at the first and fourth follow-up visits had higher socioeconomic status relative to those individuals who did not attend those visits (p<0.05). There were no significant differences in gender, race, and ethnicity between those who did and did not participate at any time point.
Data Analysis
Hierarchical linear modeling (
30) was used to assess individual intercept (initial levels) and slope (change) while accounting for the lack of independence between repeated observations of each child. Hierarchical linear modeling enables a direct-likelihood estimation of missing data (
30). Therefore, participants who do not come in at a time point but who return a year or two later are included in the analysis. Restricted maximum-likelihood estimation was used to estimate the random-effects models. Given that level 2 residuals indicated some divergence from normality in the distribution of the Mahalanobis distance, robust standard errors are reported (
31).
Factor analysis showed a single factor underlying ADHD severity and impairment at each time point. Therefore, an overall mean score of parent and teacher reports on the ADHD Rating Scale–IV and the Children’s Problem Checklist was used to indicate ADHD symptoms and impairment in the child. Similarly, the mean of the five domain scores at each time point was used to indicate neuropsychological functioning so that equal weight was provided to every domain of neuropsychological functioning. Family socioeconomic status was included as a covariate because of the association between socioeconomic status and attendance at the first and fourth follow-up visits and the significant correlation of socioeconomic status with ADHD severity.
The first model was designed to investigate trajectories of change in ADHD severity and impairment across 10 time points. Age was centered at 8 years. For instance, a child who was assessed at age 8.25 years was given a value of 0.25. This model is represented by the following equations:
Level 1 Model:
ADHDij=π0j+π1jAgeij+eij
Level 2 Model:
π0j=γ00+r0j
π1j=γ10+r1j
ADHDij is the severity of ADHD for time i for participant j; π0j and π1j are the intercept and slope, respectively, for participant j; Ageij is the age at time i for participant j; and eij is the level 1 regression residual for each participant at time i. In the level 2 equations, γ00 and γ10 indicate the average intercept and average slope, respectively. The level 2 error terms r0j and r1j signify differences between the individual and the sample average on the intercept and slope, respectively.
Model 2 similarly investigated the trajectory of change in NEPSY scores. Child age, centered at 4 years, was included as a level 1 variable. The empirical Bayes slope of change in neuropsychological functioning was saved for later use as a predictor of change in ADHD severity (see model 4 in the Results section).
To test hypothesis 1 (model 3), scores on neuropsychological functioning at baseline were used to predict change in the severity of ADHD symptoms and impairment after controlling for baseline family socioeconomic status. The level 1 model included the continuous variable of age centered at 8 years. The level 2 model included baseline neuropsychological functioning (baseline NEPSY score) and family socioeconomic status. These models are represented by the following equations:
Level 1 Model:
ADHDij=π0j+π1jAgeij+eij
Level 2 Model:
π0j=γ00+γ0jBLNEPSYj+γ02SESj+r0j
π1j=γ10+γ11BLNEPSYj+γ12SESj+r1j
Model 4 was identical to model 3 in all aspects except that the slope of NEPSY (calculated in model 2) was entered instead of baseline NEPSY score. As in the above model, the level 1 model included the continuous variable of age centered at 8 years. The level 2 model included the slope of change in NEPSY score and family socioeconomic status.
Results
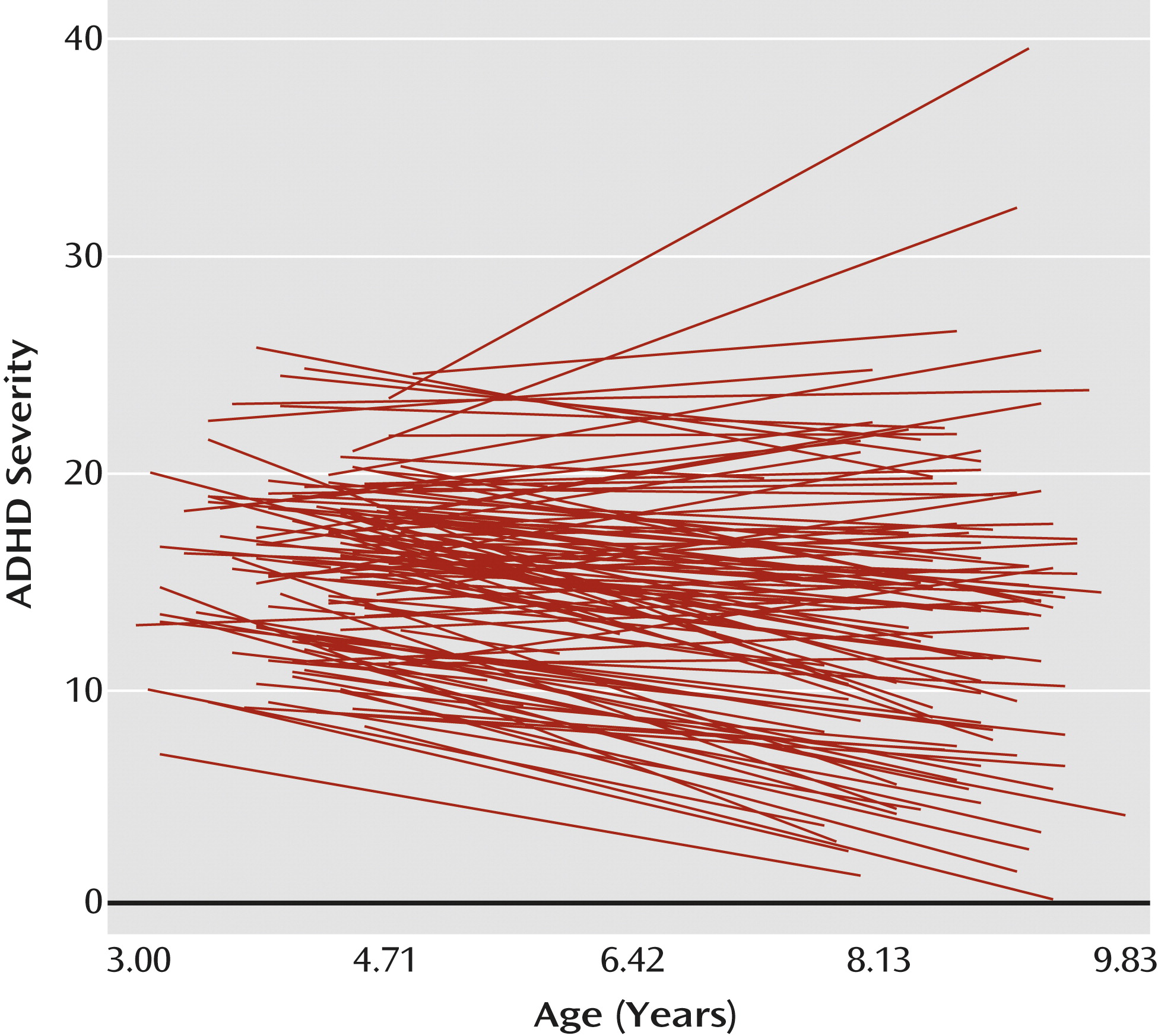
Model 1: Change in ADHD Symptoms and Impairment
The estimate of mean ADHD symptoms and impairment at age 8 years was 13.40 (SE=0.62, df=138, T ratio=21.46; p<0.001). The average slope or rate of change in ADHD symptoms and impairment at 8 years was −0.55 units (SE=0.16, df=138, T ratio=−3.45; p=0.001). There was significant variation around the average intercept (χ
2=1,044.46, df=133; p<0.001) and the average slope (χ
2=297.87, df=133; p<0.001), indicating that the children varied in their ADHD severity at age 8 and in their rate of change in ADHD severity. Therefore, both these parameters were modeled to understand the developmental trajectory of children.
Figure 1 depicts the variability in the trajectories of ADHD symptoms and impairment.
Model 2: Change in Neuropsychological Functioning
The average predicted value of neuropsychological functioning at age 4 was 94.64 (SE=0.73, df=137, T ratio=128.77; p<0.001), and this value increased by 3.11 units on average (SE=0.31, df=137, T ratio=10.00; p<0.001) with a unit increase in time (approximately every year). Moreover, there was significant variation around the average intercept (χ2=262.02, df=123; p<0.001) and the average slope (χ2=172.92, df=123; p=0.002). Overall, scores on neuropsychological functioning improved over time, although they improved less for some children than for others.
Model 3: Baseline Neuropsychological Functioning Predicting Change in ADHD Severity
Baseline neuropsychological functioning was not significantly associated with the intercept or change in ADHD severity (
Table 2).
Model 4: Change in Neuropsychological Functioning Predicting Change in ADHD Severity
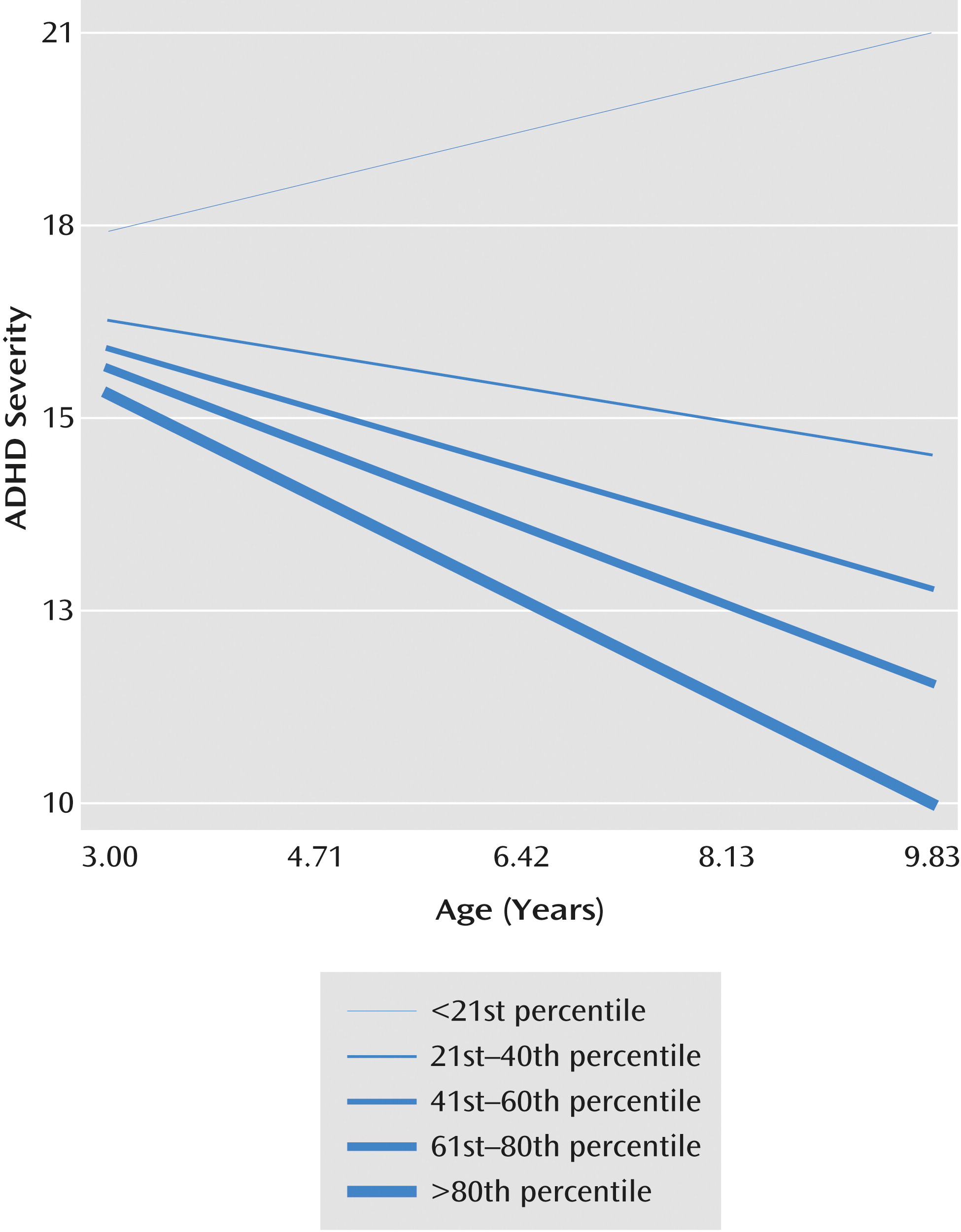
An improvement in neuropsychological functioning was significantly associated with a decrease in ADHD severity over time (
Table 3). The predicted decrease in intercept of ADHD severity at age 8 for a unit increase in neuropsychological functioning was 1.90 units. A unit change in slope of neuropsychological functioning was associated with a decrease of 0.28 units in ADHD severity.
Figure 2 depicts the association between trajectories of ADHD severity and change in neuropsychological functioning. Children who exhibited the least improvement in neuropsychological functioning had an escalation of their ADHD severity, while those who showed the greatest improvement had a decrease in ADHD severity.
Post Hoc Analysis
We ran an additional analysis with medication treatment at 8 years old (present or absent), baseline ADHD diagnostic status, and IQ as covariates (Table S2 in the online
data supplement). The slope of change in NEPSY score continued to be associated with the intercept and slope of change in ADHD severity.
Discussion
To our knowledge, this is the first study to examine the longitudinal association between overall neuropsychological functioning and trajectories of children’s ADHD symptoms and impairment during the transition from preschool to school age. Consistent with other research (
32), poorer neuropsychological functioning at baseline was not significantly associated with the intercept of ADHD severity at age 8 or with the rate of change in ADHD severity (hypothesis 1). However, improvement in neuropsychological functioning was linked to an attenuation of ADHD severity (hypothesis 2). These findings suggest that early neuropsychological status may not be etiologically linked to the subsequent trajectory of ADHD severity. Rather, change in neuropsychological functioning, irrespective of initial level, seems to track ADHD severity over development.
These findings are consistent with neuroimaging data suggesting that ADHD is characterized by delays or deficits in brain development (
9,
10) and that its remission may be marked by a normalization or improvement in neural development (
20,
33). Furthermore, these findings provide a more detailed and downward extension of data derived from adolescents and young adults who were diagnosed with ADHD in middle childhood (
15–
17). Those studies found that relative to individuals with persistent ADHD, those who had a diminution of symptoms over time performed better on an array of neurocognitive measures linked to executive functions. However, those studies did not include baseline neurocognitive assessments, making it impossible to know whether clinical improvement was associated with
change in neuropsychological functioning. Our study is unique as it links changes in neuropsychological functioning with variance in ADHD severity over and above contemporaneously collected data on ADHD severity.
Our findings are consistent with a developmental psychopathology model in which brain function (as assessed using neuropsychological tests) alters the course of ADHD during early childhood, and a diminution of ADHD symptoms and impairment across the lifespan is linked to neural development (
18). However, these data demonstrate an association, not causation. It is possible that children who become less symptomatic over time perform better on tests because decreased ADHD severity allows them to better focus on the task at hand, thus improving performance on neuropsychological tests.
This study used prospective longitudinal data from multiple sources, enhancing the validity of our findings and reducing the likelihood of reporter bias influencing the results. The study also included an impairment measure, which is required for an ADHD diagnosis but is not often included in studies assessing change in ADHD severity over time. Given that all the children in the study had impairment according to parent or teacher report, the findings are highly relevant to clinicians. The use of a dimensional approach in measuring ADHD may have enabled us to more effectively test variations in symptom severity over time. Moreover, the use of hierarchical linear modeling enabled us to account for individual variability in the initial levels and change in ADHD severity. The association of change in neuropsychological functioning with change in ADHD severity was evident even after developmental stage of the child (age), family socioeconomic status, ADHD diagnostic status, IQ, and medication treatment were factored in as covariates.
It is important to note that although all children in this study had more than six symptoms of ADHD according to parent or teacher report at baseline, some did not meet DSM-IV criteria for ADHD at the time of recruitment. Including these children and excluding asymptomatic children may have enhanced the generalizability of this study to a wider population of inattentive and hyperactive-impulsive preschool children.
Our study is not without limitations. Despite the significant association between neuropsychological functioning and ADHD severity, there was much unexplained variance, suggesting that a range of family factors (e.g., parenting relationships, marital dysfunction, maternal illness, and depression) (
34) or child temperament (
35) could have influenced the ADHD trajectories. Second, practice effects may have influenced the results, although the 1-year interval between neuropsychological assessments and age-related differences in the content of subtests diminish the likelihood that this is a significant concern. Third, our findings indicating an association between neuropsychological development and ADHD trajectory apply to young hyperactive-inattentive children, but that relationship may be different in older children, adolescents, and adults. The small number of girls in our study limited our ability to consider gender differences. Additionally, some children who were taking long-acting ADHD medications may have been in the withdrawal period of the medication at the time of testing. Finally, the average correlations between parent and teacher ratings over time were significant but modest, as observed in other research (
36).
This study employed a global measure of neuropsychological functioning instead of more specific measures of cognitive functioning for three reasons. First, ADHD is a heterogeneous disorder in which children show deficits across multiple domains of executive and nonexecutive function (
37). Second, neuropsychological functioning as measured on the NEPSY is best conceptualized as a single factor (
28). Third, neuroimaging data indicate delays in cortical development in ADHD throughout much of the neocortex, not just in one specific region (
9). Notably, our neuropsychological tasks likely engage neural networks involving both cortical and noncortical structures, making it impossible to draw conclusions about specific neural substrates associated with the attenuation of ADHD severity. Further research may try to disentangle distinct neuropsychological domains that are most closely linked to ADHD symptom reduction.
Research elucidating the factors that lower the severity of ADHD has strong public health implications given the high economic (
38), behavioral, social, and academic (
39) consequences of ADHD. The data from this study cannot shed light on the possible environmental and genetic factors influencing changes in both neuropsychological functioning and ADHD severity, and causal inferences regarding the relationship between neurocognitive and behavioral trajectories must be made with extreme caution. Nevertheless, our findings bolster the argument for assessments during early childhood and suggest that the enhancement of cognitive functioning in at-risk children (
19,
21) may have the potential to prospectively diminish ADHD severity and impairment.