Maternal depression across the postbirth period, a condition affecting approximately 15% of women in industrial societies, is a major public health concern. Not only is maternal depression among the most prevalent psychiatric disorders, but it also is the most underdiagnosed in the community and has direct negative consequences both for the suffering individual and for her child (
1). Longitudinal studies have demonstrated that children of depressed mothers show greater vulnerability to psychopathology, particularly to anxiety and depressive disorders (
2), oppositional conduct, neurocognitive deficits (
3), and maladaptive social behavior (
4). However, while theoretical models suggest that the transmission of risk from depressed mothers to their children involves both genetic transmission and dysfunctional parenting, little research has examined both genes and parent-child relatedness in order to provide an integrated view of maternal depression (
4).
Longitudinal studies of maternal depression in community samples suffer several methodological flaws that may hinder their ability to correctly estimate the effects of maternal depression or address its biological underpinnings. First, multiple contextual conditions are often associated with maternal depression, including poverty, single parenthood, teenage parenting, or premature birth (
4), and these are typically not controlled in community studies. Second, most community samples use maternal self-reports. However, since depression alters the individual's negative attributions, exclusive use of self-reports may be less accurate than clinical diagnoses (
5). Finally, children's social outcomes are typically not included in longitudinal studies, and direct observations of child social behavior in real-life situations beyond infancy rarely exist.
One neurobiological system that may underlie the development of maternal depression and its cross-generational effects is the oxytocin system. Oxytocin, a nine-amino-acid neuropeptide synthesized in the hypothalamus, has long been implicated in birth and lactation, and recent research highlighted its role in providing the neurohormonal substrate for mammalian social bonding (
6). Animal studies have shown that the neurochemical organization of infant brain oxytocin is shaped in early life through patterns of maternal behavior, such as licking and grooming (
7). This extragenomic cross-generational transmission defines a biobehavioral feedback loop: maternal oxytocin determines the mother’s caregiving behavior, which in turn shapes the infant's oxytocin through species-typical parenting behavior (
8). Human studies similarly demonstrate the involvement of oxytocin in parenting behavior, particularly social synchrony and affectionate touch (
9), and show that parental oxytocin influences the infant's oxytocin system through the provision of synchronous parenting (
10). Oxytocin administration to a parent increases salivary oxytocin production and social engagement in parent and child (
11), and synchronous mothers display greater brain activations in response to infant stimuli in oxytocin-rich brain areas (
12). Similarly, oxytocin is linked with empathy, theory of mind, and prosocial orientation (
13), pointing to its role in the general capacity for collaborative engagement with the social world (
6).
Numerous studies have shown disruptions to the oxytocin system in depression, and thus, addressing its role in maternal depression may be a fruitful area of research and potential intervention. Plasma oxytocin levels are lower in patients with major depression (
14) and inversely correlate with depressive symptoms in depressed patients (
15) and healthy adults (
16). Plasma oxytocin during the third trimester of pregnancy predicts maternal postpartum depressive symptoms (
17), and lower plasma oxytocin in the first trimester predicts postpartum depressive symptoms and a low level of attachment behavior (
6). Overall, depressed mothers exhibit little social synchrony and minimal affectionate touch (
18). Since these behaviors provide critical inputs for the infant's brain oxytocin, their elimination may result in less functional child oxytocin, in addition to genetic liability. Given the limitations of current theories of depression and anxiety, which emphasize the roles of serotonin and norepinephrine, research on oxytocin may provide a new direction for understanding these conditions.
In addition, neurogenetic studies have shown that allelic variations on the oxytocin receptor gene,
OXTR, are associated with both prosocial tendencies and disorders involving disruptions to social functioning (
19). Brüne (
20) suggested that the A allele of the
OXTR rs2254298 polymorphism is more evolutionarily recent and may be viewed as a “plasticity allele,” conferring resilience under nonoptimal early conditions. The rs2254298 G allele is related to greater risk for autism (
21) and major depression, with GG homozygous carriers (versus AG and AA) more likely to report avoidant attachment (
22). The presence of a single A allele has been associated with greater attachment security in children (
23), and parents carrying the A allele (AG or AA genotype) had higher plasma oxytocin levels and exhibited more social synchrony and affective touch during interactions (
24). It is thus possible that while the oxytocin system may be disrupted in depression, the
OXTR rs2254298 A allele may chart greater resilience in the context of early and chronic exposure to maternal depression.
The overall goal of the current study was to test the effects of maternal depression across the child's early years on child psychopathology and social outcomes and to address the role of oxytocin in maternal depression. Utilizing an extreme-case design, we recruited a large community cohort of mothers with no contextual risk at birth and assessed depressive symptoms three times across the postpartum year. At 9 months and again at 6 years, the mothers were assessed for diagnoses of major depressive disorder. This method resulted in two matched cohorts of chronically depressed versus never-depressed mothers, a design fruitfully used to study the cross-generational effects of oxytocin and parenting in animal models (
25). We hypothesized that 1) chronic maternal depression would markedly increase the child’s propensity for psychopathology, 2) the child’s levels of social engagement and empathy would be lower in the clinical group, 3) the oxytocin system would be disrupted in family members of depressed mothers, as reflected in lower peripheral oxytocin levels, and 4) the presence of the
OXTR rs2254298 A allele in mothers would confer resilience in the context of chronic exposure to maternal depression.
Method
Participants
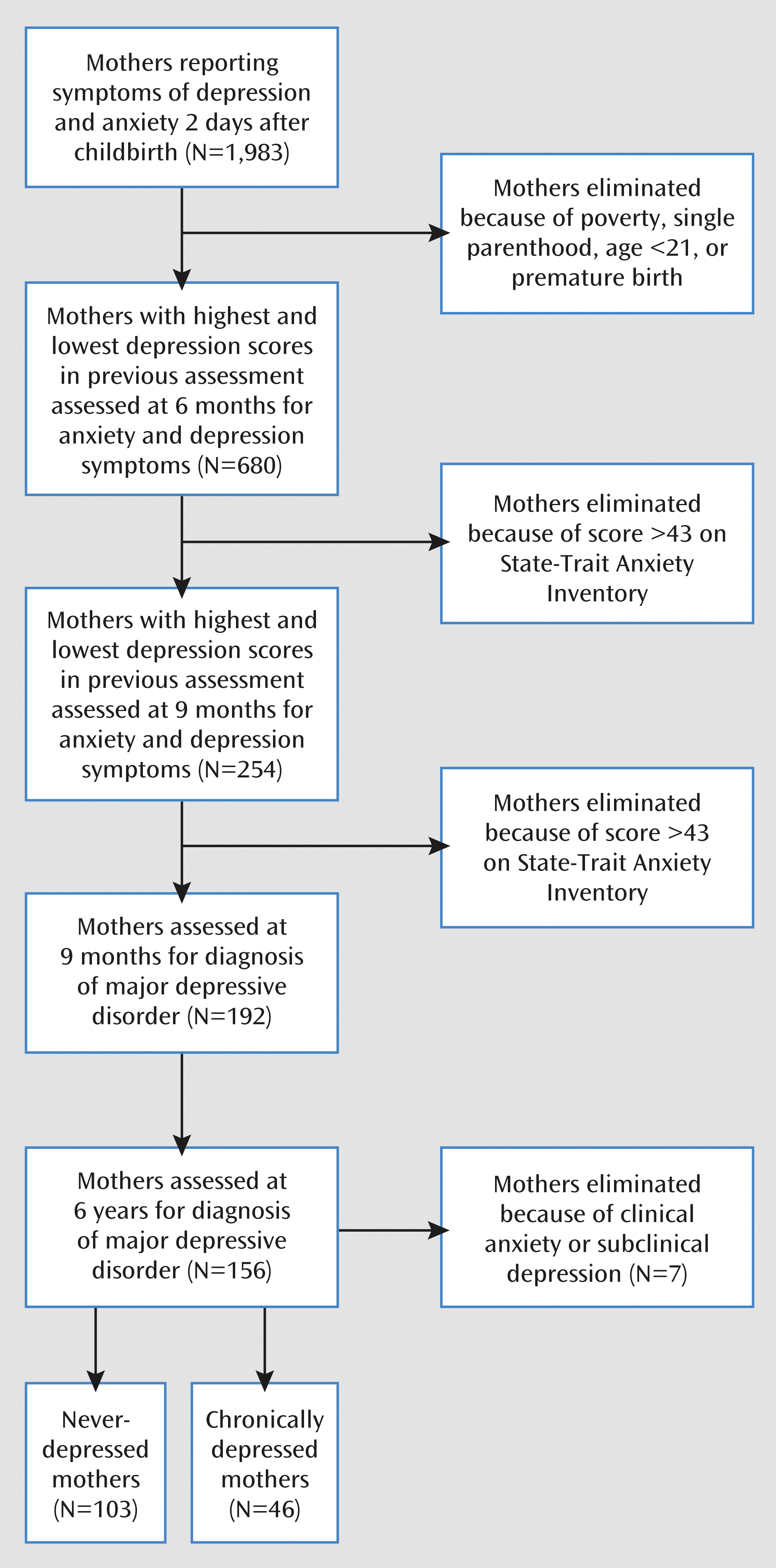
The initial cohort comprised 1,983 women who were recruited on the second postbirth day in three maternity wards and completed measures of anxiety and depression. The study included only mothers who were healthy, had completed high school, were at least 21 years old, and were married or cohabitating and whose infants were born at term (>36 weeks gestation, the Israeli cutoff for admission to preterm unit), healthy, and singleton. Their families were all above the poverty line. Women with Beck Depression Inventory (
26) scores in the highest and lowest quartiles completed measures of anxiety and depression at 6 months (900 were approached and 680 responded, 76%) and again at 9 months (350 were approached and 254 responded, 73%), as shown in
Figure 1. Women with high levels of anxiety symptoms (State-Trait Anxiety Inventory score above 43) were excluded. Of the responding mothers at 9 months, 192 (76%) were clinically diagnosed and observed. Of these women’s families, 156 (81%) including mothers, fathers, and children were visited when the child was 6 years old. The children’s mean age was 6.33 years (SD=1.25), the mother's age was 38.66 years (SD=4.40), and the father's age was 41.04 years (SD=4.74). Eighty percent of the parents had college degrees, 91% were married, and 89% of the mothers were employed. Among the children, 51% were boys and 36% were the firstborn. Of the mothers diagnosed with depression (see following section), two (4%) were treated with medication, and four depressed mothers (9%) and 10 comparison mothers (10%) received psychotherapy, with no effect on outcome. At 6 months, 42% of the comparison mothers and 11% of the depressed mothers breast-fed (χ
2=15.34, df=1, p<0.001); at 9 months, 15% of the comparison mothers and 2% of the depressed mothers breast-fed (χ
2=23.80, df=1, p<0.001).
The study was conducted in accordance with the Declaration of Helsinki, and all procedures received the approval of the institutional review board of Bar-Ilan University. All procedures were explained to the adult participants before the beginning of the study, and all of them signed informed consent statements. For their participation in the study, the parents received a gift certificate of 250 new Israeli shekels (NIS), approximately $70 U.S.
Psychopathology in Family Members
The families were visited at home in the afternoon or evening to control for diurnal variability in oxytocin (
9), and each visit lasted approximately 4 hours.
The mothers were evaluated with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (
27). Forty-six mothers (29%) were defined as chronically depressed (
Figure 1). These mothers showed a high level of depressive symptoms (a score above 11 on the Beck Depression Inventory) at childbirth, 6 months, and 9 months, received a diagnosis of major depressive disorder at both 9 months and 6 years, and reported being depressed throughout the child's first 6 years. Similarly, 103 mothers (66%) were defined as comparison subjects, showing no elevated symptoms at any time point and not receiving any axis I diagnosis in either assessment. Seven mothers were excluded because of clinical anxiety (N=3) or subclinical depression (N=4).
The children were diagnosed with the Development and Well-Being Assessment. This is a structured interview and questionnaire that generates ICD-10 and DSM-IV diagnoses in children 4–16 years old (
28). It has been well validated in a large epidemiological study in Israel (
29) and other investigations. The diagnostic assessments were conducted by four clinical psychologists (including Y.A.L., M.F., and A.V.) supervised by a child psychiatrist, all of whom were blind to all other information.
The father's depressive and anxiety symptoms were assessed with the Beck Depression Inventory and State-Trait Anxiety Inventory. The scores were standardized and summed to create an overall measure of emotional distress.
Salivary Oxytocin and OXTR Genotype
Saliva was collected by means of Salivette (Sarstedt, Rommelsdorft, Germany). Parents and children were asked to chew a roll of cotton for 1 minute. The saliva samples were kept ice-chilled for up to 1 hour before being centrifuged at °4C at 1500
g for 15 minutes. The liquid samples were stored at 80°C. To concentrate the samples by three or four times, the liquid samples were lyophilized overnight and kept at 20°C until assayed. Dry samples were reconstructed in the assay buffer immediately before analysis by enzyme immunoassay, as in previous research (
10,
11). Oxytocin level was determined with a commercial oxytocin enzyme-linked immunosorbent assay kit. Measurements were performed in duplicate, and concentrations were calculated by means of MatLab 7 (MathWorks, Natick, Mass.) according to standard curves. Intra-assay and interassay coefficients were <12.4% and 14.5%, respectively.
For genotyping, DNA from the mother, father, and child was extracted from 20 ml of mouthwash samples by means of the Master Pure kit (Epicentre, Madison, Wis.). Genotyping of
OXTR was performed by the SNaPshot method (Applied BioSystems, Foster City, Calif.) as previously described (
24). All polymerase chain reaction (PCR) and high-resolution melting analyses were performed on a Rotor-Gene 3000 DNA amplification system (Corbett Life Science, Sydney, Australia), by using the following primers, which produced a 162-base-pair amplicon: F5′GGTGCACAGACCACTTAGCA′3; R5′TCGGAAGAGAGGAAAGCAAA′3. PCR reaction conditions were as follows: activating enzyme step at 95°C for 15 minutes, 45 cycles of denaturation at 95°C for 5 seconds, reannealing at 60°C for 15 seconds, and extension at 72°C for 10 seconds. The reaction proceeded to a hold at 40°C for 2 minutes and a second hold at 82°C for 2 minutes, and then the melt procedure ramped from 82°C to 90°C, raising by 0.1°C every 3 seconds, where fluorescence was acquired. High-resolution melting distinguished between the three genotypes (AA, AG, GG), and the method was verified by comparison of a portion of the high-resolution melting results to those obtained by genotyping the same samples by means of the SNaPShot procedure described in the preceding. Frequencies of the
OXTR genotypes were in Hardy-Weinberg equilibrium. DNA concentrations above 5 ng/μl were available from 98% of the samples and successfully genotyped in 96%.
Mother and Child Behaviors
Ten minutes of mother-child interactions with preselected toys were filmed (
18). Interactions were coded with the Coding Interactive Behavior manual, a well-validated system with good psychometric characteristics (
30). Interrater reliability on 20% of the study group exceeded 90%. The construct assessing child social engagement was used and included the child’s social gaze, positive affect, initiation of social bids, competent use of environment, and creative-symbolic play (alpha=0.76). Additionally, the four behavioral markers of human mothering were coded: social gaze, positive affect, warm vocalizations, and affectionate touch.
Child empathy was coded from four observational situations that exposed the child to the experimenter's feigned distress: coughing painfully, hurting a foot, sighing sadly, and crying. Each episode was coded on multiple scales (rated 1–5) with reliability above 90%. The empathy construct was the average of the ratings of “empathic concern” and “maintaining involvement” from the four experimental observations. All coding was conducted by two graduate students in psychology who were blind to all other information.
Statistical Analysis
Distributions of axis I disorders in the children of depressed and nondepressed mothers were examined with chi-square analyses. Multivariate analysis of covariance (MANCOVA), with fathers' emotional distress as covariate, examined group differences in 1) child social outcomes (empathy, social engagement) and 2) salivary oxytocin levels in family members. Multivariate analysis of variance examined differences in salivary oxytocin between mothers carrying the GG genotype and those with the AG or AA genotype. Univariate analysis of variance (ANOVA) examined the effects of maternal depression and OXTR genotype on child psychopathology.
Results
Maternal Depression and Child Psychopathology
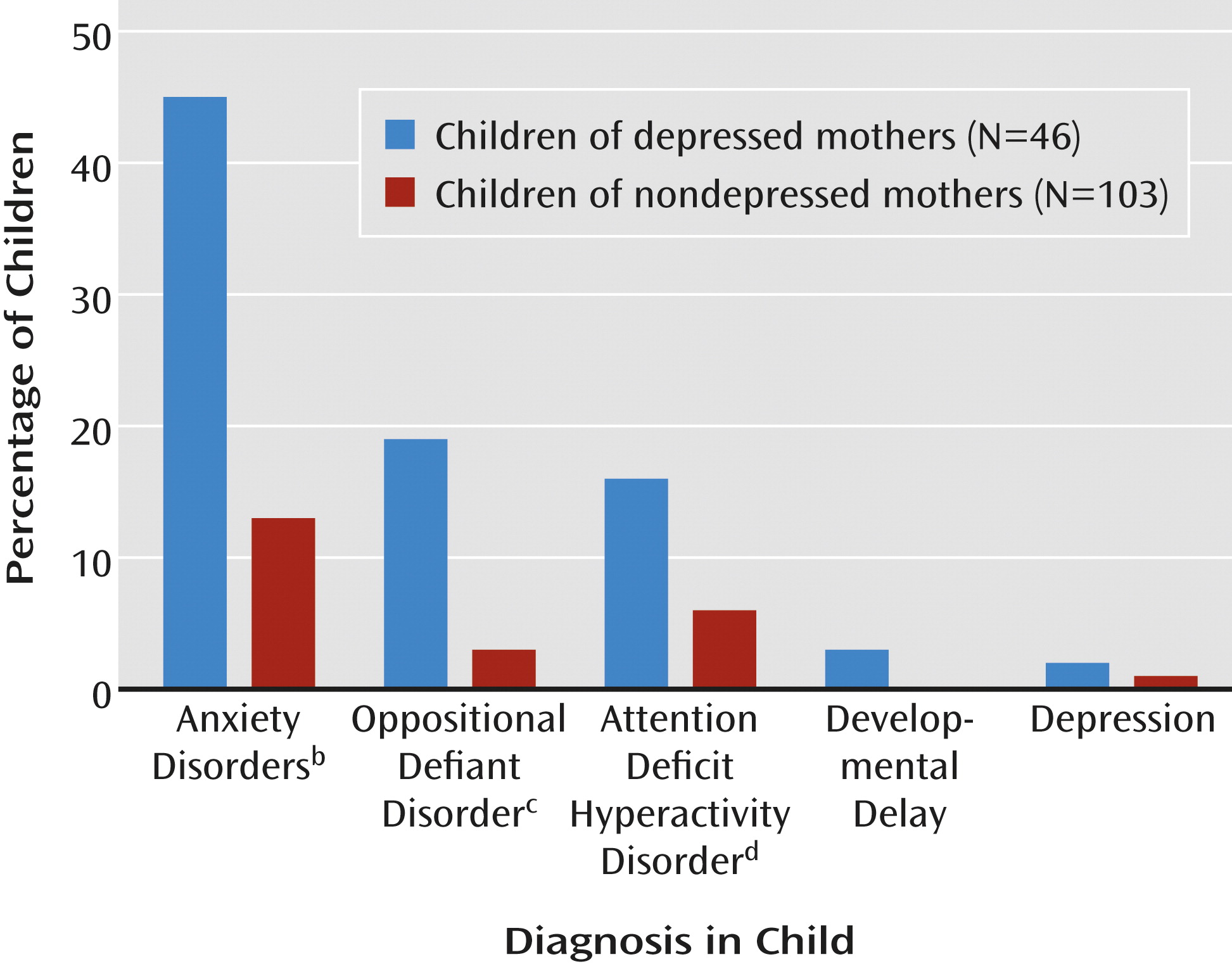
Chronic maternal depression increased the prevalence of child psychopathology by fourfold, a significant difference (
Figure 2). Among the children of depressed mothers, 28 (61%) received axis I diagnoses, whereas among children of nondepressed mothers, only 15 (15%) received clinical diagnoses. The prevalences of anxiety disorders, oppositional defiant disorder, and attention deficit hyperactivity disorder were also higher in the children of the depressed mothers (
Figure 2).
Maternal Depression and Child Social Outcomes
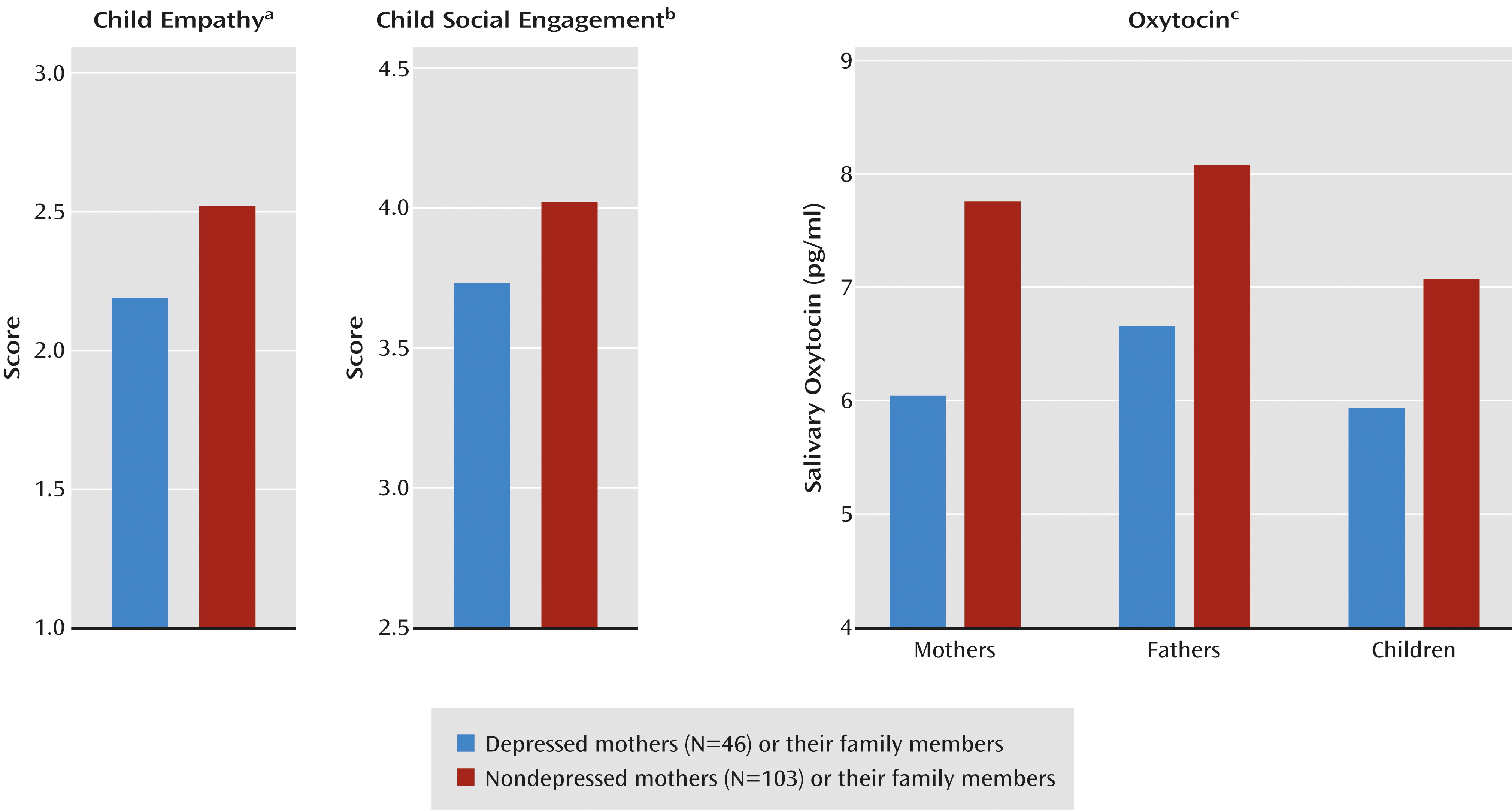
MANCOVA showed an overall effect of maternal depression on the children's social outcomes (F=5.86, df=2, 145, p=0.003) and no effect of emotional distress in the father. The univariate tests showed lower levels of social engagement and empathy in the children of the depressed mothers (
Figure 3).
Maternal Depression and Oxytocin
MANCOVA showed overall effects of maternal depression on the salivary oxytocin levels of family members (F=4.07, df=3, 143, p=0.009) and an overall effect of the father’s emotional distress (F=7.20, df=3, 143, p<0.001). Univariate tests showed lower salivary oxytocin levels in the mothers, fathers, and children (
Figure 3). Oxytocin levels were interrelated between mothers and children (r=0.47, N=298, p<0.001), between fathers and children (r=0.23, N=298, p=0.006), and between mothers and fathers (r=0.35, N=298, p<0.001).
The depressed mothers were more likely to have the GG risk genotype for OXTR rs2254298 than were nondepressed mothers (67% versus 34%; χ2=12.97, df=1, p=0.001). The GG genotype was also more common among the children (62% versus 39%; χ2=8.82, df=1, p=0.02) and husbands (59% versus 37%; χ2=7.89, df=1, p=0.02) of depressed mothers. Mothers with the AA or AG genotype provided more affectionate touches (mean score=1.79, SD=0.96) than mothers with the GG genotype (mean=1.42, SD=0.65) (F=3.64, df=1, 148, p<0.05). Maternal touch correlated with the mother's salivary level of oxytocin (r=0.16, N=149, p=0.05) and child social engagement (r=0.18, p=0.03). Child social engagement correlated with the child’s oxytocin level (r=0.17, N=149, p=0.04).
MANOVA of the maternal OXTR rs2254298 genotype, GG versus AA or AG, showed an overall effect of genotype (F=3.06, df=3, 143, p=0.04), indicating that family members of mothers with the GG genotype had lower salivary oxytocin levels. Univariate tests showed lower salivary oxytocin levels in these mothers (F=4.71, df=1, 145, p=0.04), their husbands (F=3.96, df=1, 145, p<0.05), and their children (F=6.52, df=1, 144, p=0.02).
Moderating Effects of OXTR Genotype
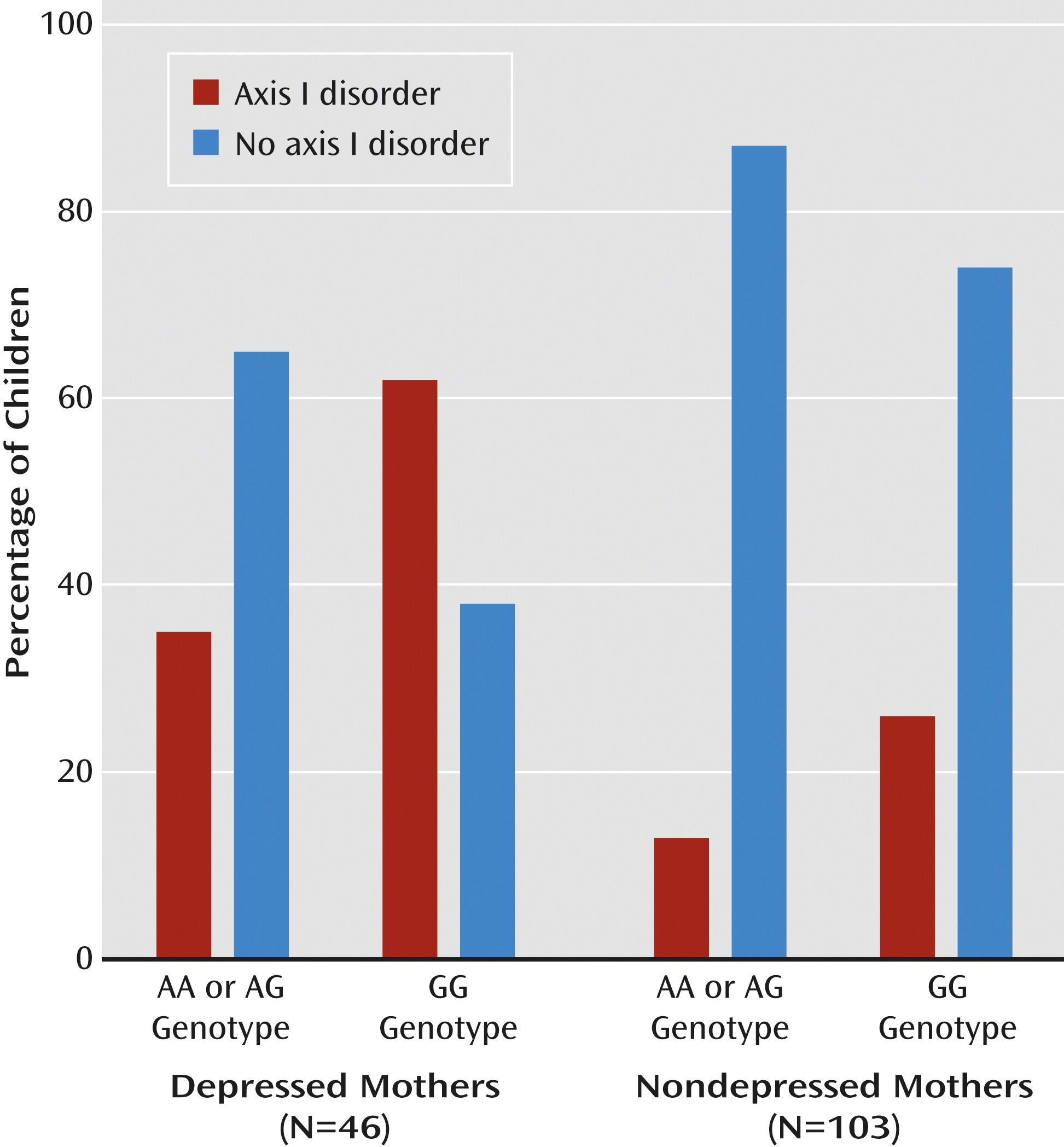
Finally, we tested whether
OXTR buffers the effects of maternal depression on child psychopathology. Univariate ANCOVA showed a main effect for maternal depression (F=4.41, df=1, 145, p=0.04) and a main effect for the
OXTR rs2254298 GG versus AA or AG genotype (F=4.36, df=1, 145, p=0.04) but not for the father’s symptoms. These findings indicate that children of depressed mothers and of mothers with the
OXTR rs2254298 GG genotype were more prone to psychopathology. An interaction between maternal depression and
OXTR genotype also emerged (F=7.18, df=1, 145, p=0.006). Among the 46 depressed mothers, 29 (63%) were GG homozygous, whereas 17 were GA or AA (37%). Among the depressed GG mothers, 18 of their children showed axis I disorders (62%) and 11 did not (38%). However, among the depressed mothers carrying one or two A alleles, i.e., with either the GA or AA genotype, risk was cut by nearly half: of these 17, only six children (35%) exhibited axis I disorders, while 11 (65%) did not. Within the nondepressed group, a similar profile emerged, although only 35% of the mothers (N=36) had the GG genotype, and 65% had GA or AA (N=67). Of the 36 children of nondepressed GG carriers, eight had axis I disorders (22%) while 28 did not (78%). This risk was reduced by half among A-carrying (GA or AA) mothers, with seven children (10%) showing psychiatric disorders and 60 (90%) not (
Figure 4). No child outcome correlated with maternal or paternal age. Maternal breast-feeding was unrelated to outcome, did not moderate the effects of depression on outcome, and was unrelated to
OXTR distributions.
Discussion
This study is among the first to follow outcomes of maternal depression from the birth of the child while controlling for contextual risk typically associated with maternal depression. The study is also unusual in including fathers and measuring genetic and hormonal biomarkers from mother, father, and child. We found that by school entry, children growing up in the context of chronic maternal depression from birth were four times as likely to develop axis I disorders as were the children of nondepressed mothers. These children showed less social engagement and less empathy in response to the distress of others as measured by direct observations of behavior in daily situations. Overall, the findings suggest that continuous exposure to maternal depression bears long-term negative consequences for child development, regardless of contextual risk. We further found that the oxytocin system is dysfunctional in family members of chronically depressed mothers, as indicated by a lower peripheral oxytocin level and higher genetic risk related to the OXTR gene. Finally, the presence of the OXTR rs2254298 plasticity A allele appears to confer resilience in the context of maternal depression and was associated with a marked reduction in the child’s propensity for psychopathology.
Oxytocin has long been linked with birth and lactation. The recent surge of studies highlighting its involvement in attachment and sociocognitive processes indicates that humans, like other mammals, learn to function within their ecosocial niche on the basis of biobehavioral experiences occurring within the mother-infant bond (
6). Oxytocin is among the systems most sensitive to epigenetic effects and is shaped in postnatal life through patterns of maternal care (
9,
10). Such an environment-sensitive birth-related system is a natural candidate as an underlying mechanism of maternal depression. We found that the less malleable GG genotype was overrepresented in family members of depressed mothers, demonstrating, for the first time to our knowledge, a relationship of genetic risk in the oxytocin system to maternal depression and its cross-generational influence.
Similarly, depressed mothers, fathers, and children had lower peripheral oxytocin levels than the members of families of nondepressed mothers (10). Since the OXTR rs2254298 GG genotype is linked with lower peripheral oxytocin levels and less parenting behavior in both fathers and mothers (13), the children of depressed mothers were growing up in low-oxytocin environments with little compensatory input from their fathers. Studies have underscored the links among disruptions in oxytocin, lower empathy levels, and disorders of social dysfunctions (31), and the current findings suggest that the consistency of oxytocin dysfunction within families may contribute to the cross-generational transfer of psychiatric vulnerability.Gene-by-environment interaction provides the framework for much current research in psychiatry (
32), and models emphasize the notion of plasticity genes that may confer resilience in nonoptimal rearing contexts (
33). The
OXTR rs2254298 AG or AA genotype has been suggested as indicating greater plasticity and more resilience to negative early environments (
20). Presence of the rs2254298 A allele has been linked to bilateral amygdala volume, with AA carriers showing the greatest and GG the smallest amygdala size (
34). The observations that amygdala size correlates with the size of the individual's social network (
35) and that the complexity of the social network relates to brain size in primates (
36) suggest that the A allele may have an evolutionary advantage by increasing the capacity to respond in real time to subtle social signals, i.e., social synchrony. Consistently, we found that A-carrying (AA or AG) mothers had higher peripheral oxytocin levels, which correlated with a higher oxytocin level and better social outcomes in the child. To our knowledge, these findings are the first to demonstrate the cross-generational transfer of psychiatric vulnerability through an interchange between genetic risk and critical mammalian parenting behavior.
Our findings have important implications for social policy and the potential for pharmacological intervention in maternal depression. The fact that 60% of children growing up with chronically depressed mothers showed axis I disorders by school entry, even without contextual risk, should raise concern among health care policy makers. From a pharmacotherapy perspective, the findings suggesting that a more functional oxytocin system even in the context of chronic maternal depression provides a buffer against the cross-generational transfer of vulnerability and opens the possibility of an “oxytocinergic” therapeutic window. The provision of affectionate touch increases maternal oxytocin production (
6), highlighting the possibility of touch-based interventions, such as massage therapy, which has been fruitfully applied in postpartum depression (
37). Since depressed mothers provided less breast-feeding and the reduction in breast milk and bodily contact may have negatively affected the infant’s oxytocin level, such interventions may be useful. Oxytocin has been tested as a psychopharmacological agent in disorders involving social dysfunction, including autism, schizophrenia, and social anxiety, with mostly favorable results (
38), and could be implemented in maternal depression. The finding that oxytocin administration to a parent increased oxytocin production in parent and child (
11) suggests that administration to the mother may be sufficient to boost the infant's oxytocin level during its critical period.
Several study limitations should be considered. First, although comorbidity of depression and anxiety was common, we chose to eliminate mothers with comorbid anxiety disorders or high levels of anxiety symptoms as a first step. Research has documented opposite trends for peripheral oxytocin levels in the two disorders—low in depressed women and high in anxious women—and depressed and anxious mothers present opposite parenting styles (
39). Prenoveau et al. (
40) found that in the context of motherhood, generalized anxiety disorder and depression represent different states, and neuroimaging studies have discriminated the two disorders (
41). However, as anxiety is the most prevalent comorbid condition in major depression, our findings pertain to a subgroup of depressed mothers and future research is necessary to examine the generalizability to mothers with both conditions. The extreme-case design has advantages in large community samples, is informative from a public health perspective, and follows animal research on the cross-generational transmission of oxytocin and parenting. However, although the response rates at each stage were high (75%−82%) and attrition was randomly distributed between the chronically depressed and never-depressed mothers, those with high current symptom levels may have declined participation. Our focus on maternal depressed mood throughout the first year precluded clinical diagnosis in the first postpartum month, and future research is required to fill this gap.
Oxytocin functions within a multifaceted biobehavioral milieu, and future research should examine its interaction with other neurochemical systems, such as the dopamine and glucocorticoid systems. Oxytocin reduces stress, increases social motivation, and interacts with the stress, reward, and immune systems (
8). Since stress management and social motivation are learned within the mother-infant bond, studies targeting their joint involvement in the cross-generational transfer of psychopathology are necessary. Future studies should focus on developing better diagnostic tools for early identification of maternal depression along with short home-based interventions. Finally, research should relentlessly search for biomarkers, behavioral patterns, and relationships beyond the parent-child bond that may confer resilience in the context of maternal depression in order to provide these children a better opportunity to optimally adapt to their social world.