Varenicline, a nicotine receptor partial agonist, is approved by the U.S. Food and Drug Administration (FDA) for use in smoking cessation. Rates of smoking cessation with varenicline are generally two to three times greater than in unassisted quit attempts (
1,
2). Postmarketing surveillance evidence that varenicline may be associated with a risk of neuropsychiatric events, such as depression and suicidal thoughts and behavior, led the FDA in 2009 to issue a black box warning (
3).
The safety of varenicline with regard to neuropsychiatric events and possible suicide has been debated, thus far without resolution (
4). Although higher frequencies of spontaneous reports of depression and suicide and related thoughts and behaviors have been reported for varenicline (
5), randomized controlled trials have not shown evidence of an elevated risk (
2). For example, a pooled analysis of 10 of the Pfizer randomized controlled trials (through 2008) (
6) found a relative risk of 1.02 (95% CI=0.86–1.22) for incidence of psychiatric disorders (other than sleep disturbance) for varenicline (N=3,091) relative to placebo (N=2,005) and no cases of suicidal ideation or behavior. Similarly, Garza et al. (
7), studying 110 smokers with no history of psychiatric illness in a placebo-controlled randomized controlled trial of varenicline, found no effect of varenicline on measures of depression, anxiety, aggression, or irritability. In a large-scale observational study, Gunnell et al. (
8) examined reports of depression and suicidal thoughts and behavior in patients taking nicotine replacement therapy, varenicline, or bupropion in a cohort of over 80,000 patients in the United Kingdom and found no evidence of an effect of varenicline on these neuropsychiatric events relative to bupropion and nicotine replacement therapy. Kasliwal et al. (
9), using physician survey-based prescription-event monitoring to study the adverse event profile of varenicline in a cohort of 2,682 patients, found two cases of attempted suicide during treatment in patients with a history of psychiatric illness. A recent randomized controlled trial (
10) of 294 community volunteers comparing varenicline and bupropion to placebo in terms of prospective neuropsychiatric endpoints found decreased ratings of depression, negative affect, and sadness in the varenicline group relative to the placebo group.
The FDA commissioned the Department of Veterans Affairs (VA) and the Department of Defense (DOD) to conduct large-scale observational studies to compare the risk of psychiatric hospitalization between patients treated with varenicline and those treated with nicotine replacement therapy (
11). These two parallel studies used propensity score matching (
12) to minimize bias related to the differential selection effects for the two treatments. Although the FDA reported that both studies found no difference in risk of neuropsychiatric hospitalizations between varenicline and nicotine replacement therapy, it also noted that the studies had several limitations. For one thing, the studies were restricted to neuropsychiatric events requiring hospitalization; thus, for example, a suicide attempt that led to treatment limited to the emergency room would not have been included in this analysis. Second, the studies were not sufficiently powered to detect rare adverse events. The published paper on the DOD study (
13) contains more details regarding the study design, analysis, and results and reports no increase in neuropsychiatric hospitalization rate in the varenicline group relative to the nicotine replacement therapy group during the periods of either 30 days or 60 days following a new treatment initiation (
13). The study was conducted prior to the first FDA warning, thus minimizing selection effects and the effect of stimulated reporting of neuropsychiatric adverse events.
To further study the question of the safety of varenicline in terms of neuropsychiatric events, we obtained all patient-level longitudinal efficacy and safety data (covering depression, aggression/agitation, suicidal events, and nausea) from the 17 randomized placebo-controlled trials of varenicline conducted by Pfizer. These studies include two recent trials conducted exclusively in patients with a recent history of depression or schizophrenia, so we were able to examine the impact of current psychiatric disorder or history of psychiatric illness on the effect of treatment on neuropsychiatric events. Through the Freedom of Information Act, we obtained complete neuropsychiatric event data from the DOD study of neuropsychiatric events in 35,800 outpatients and inpatients treated with varenicline or nicotine replacement therapy (
13). The DOD data allowed us to determine the generalizability of the clinical trial findings to a general population sample that was not subject to the inclusion and exclusion criteria characteristic of randomized controlled trials. These data also allowed us to present in more detail the results of all events (outpatient and inpatient) than were previously reported by the original investigators (
13).
Method
Study Data
Randomized controlled trials.
A detailed description of the study data is provided in
Table 1. Altogether, the 17 studies included 4,823 participants treated with varenicline, 795 treated with bupropion, and 3,204 who received placebo, for a total of 81,105 weekly measurement occasions during active treatment. Average treatment duration was 11.6 weeks. A total of 1,004 patients had a current psychiatric disorder (studies A3051072 and A3051122) or past psychiatric illness (a subset of patients in the other 15 studies), including depression, schizophrenia, psychosis, panic disorder, anxiety disorders, mood disorders, and personality disorders. We examined both the outcome of suicidal thoughts and behavior that formed the basis for the black box warning and the possible causal factors of depression, aggression, agitation, and mood lability, as well as the unrelated side effect of nausea as a positive control. Our measure of depression was the Medical Dictionary for Regulatory Activities (MedDRA) high-level safety group term “depressed mood disorders and disturbances,” consisting of agitated depression, anhedonia, depressed mood, depression, depressive symptoms, dysthymic disorder, feeling of despair, and major depression. Aggression/agitation was defined as the combination of the lower-level MedDRA terms aggression, aggressive, aggressiveness, aggressivity, aggressivity signs, agitation, emotional lability, feelings of aggression, hostility, increased agitation, labile emotions, labile mood, labile moods, observed aggressiveness, and violent behavior. Suicidal events were defined as suicidal thoughts and behavior. Nausea included all lower-level MedDRA terms that included the word nausea.
Observational study.
As described by both the FDA (
11) and the original investigators (
13), the DOD study was a retrospective cohort study comparing acute (30-day and 60-day) rates of neuropsychiatric adverse events with varenicline or nicotine replacement therapy, using data from the Military Health System. The data were from a period before the FDA warnings were issued (August 1, 2006, to August 31, 2007), so selection effects and stimulated reporting resulting from the warnings were minimized. Overall, 19,933 patients were treated with varenicline and 15,867 with nicotine replacement therapy (nicotine patch). The data were restricted to new users, defined as individuals who had not received treatment with either varenicline or nicotine replacement therapy for the past 180 days. Following the original study (
13), we examined the following neuropsychiatric diagnoses: “anxiety disorder, depressive disorder, drug-induced mental disorders, transient mental disorders, schizophrenia, episodic and mood disorders, delusional disorders, other psychiatric disorder, anxiety disorders, posttraumatic stress disorder (PTSD), and suicide attempt.” Data analyses were conducted that both included and excluded patients with a current psychiatric disorder or a history of psychiatric illness.
Statistical Methods
Randomized controlled trials.
Efficacy data were analyzed using a three-level mixed-effects logistic regression model (
14). Week was expressed as log
e(week) to accommodate nonlinearity in temporal response patterns. The treatment-by-time interaction describes the effect of treatment on the rate of smoking over time. Comparisons were made to both placebo and bupropion. For adverse event rates (depressed mood disorders and disturbances; suicide; aggression; nausea), data were collapsed over time and adverse event rates were compared between varenicline and placebo (bupropion was not included) using a two-level mixed-effects logistic regression with random intercept and treatment effects. Nausea was included as a positive control, since varenicline has been documented to produce nausea in some patients. Both an overall analysis and an analysis examining the treatment-by-psychiatric illness interaction were performed. A second analysis compared rates of depressed mood disorders and disturbances following treatment discontinuation. Analyses were conducted using SuperMix (
15).
Observational study.
Unadjusted rates of neuropsychiatric disorders were compared between varenicline and nicotine patch using Fisher’s exact test. Propensity score matching was used to create a 1:1 matched set of varenicline and nicotine patch patients in terms of demographic characteristics, historical (past-year) comorbid physical illness and psychiatric illnesses, and psychiatric and smoking cessation medications (
Table 2). Rates were compared using generalized estimating equations (
16).
Results
Randomized Controlled Trials
Suicidal thoughts and behavior.
The overall effect of varenicline treatment on suicidal thoughts and behavior was not significant (odds ratio=0.57, 95% CI=0.23–1.38). Psychiatric illness did not moderate the effect of varenicline treatment. The rate of suicidal thoughts and behavior in patients with no current psychiatric disorder or history of psychiatric illness was 1.46 per 1,000 for placebo patients, compared with 0.47 per 1,000 for varenicline patients. The rate of suicidal thoughts and behavior in patients with a current psychiatric disorder or a history of psychiatric illness was 15.39 per 1,000 for placebo patients, compared with 14.57 per 1,000 for varenicline patients. There were no suicides.
Depression.
The overall effect of varenicline treatment on depression was not significant (odds ratio=1.01, 95% CI=0.68–1.52). Psychiatric illness did not moderate the effect of varenicline treatment. The rate of depressed mood disorders and disturbances in patients with no current psychiatric disorder or history of psychiatric illness was 20.02 per 1,000 for placebo patients, compared with 22.70 per 1,000 for varenicline patients. The rate of depressed mood disorders and disturbances in patients with a current psychiatric disorder or history of psychiatric illness was 81.32 per 1,000 for placebo patients, compared with 80.15 per 1,000 for varenicline patients. No effect of varenicline treatment on depressed mood disorder and disturbance events was observed following treatment discontinuation (rates were 3.45 per 1,000 for placebo patients and 3.08 per 1,000 for varenicline patients; odds ratio=0.76, 95% CI=0.21–2.72).
Aggression/agitation.
The overall effect of varenicline treatment on aggression/agitation was not significant (odds ratio=1.27, 95% CI=0.85–1.92). Psychiatric illness did not moderate the effect of varenicline treatment. The rate of aggression/agitation in patients with no current psychiatric disorder or history of psychiatric illness was 6.91 per 1,000 for placebo patients, compared with 9.83 per 1,000 for varenicline patients. The rate of aggression/agitation in patients with a current psychiatric disorder or history of psychiatric illness was 46.15 per 1,000 for placebo patients, compared with 51.00 per 1,000 for varenicline patients.
Nausea.
The overall effect of varenicline treatment on nausea was significant (odds ratio=3.69, 95% CI=3.03–4.48). Psychiatric illness did not moderate the effect of varenicline treatment. The rate of nausea in patients with no current psychiatric disorder or history of psychiatric illness was 92.80 per 1,000 for placebo patients, compared with 275.45 per 1,000 for varenicline patients. The rate of nausea in patients with a current psychiatric disorder or history of psychiatric illness was 109.89 per 1,000 for placebo patients, compared with 315.12 per 1,000 for varenicline patients.
Efficacy.
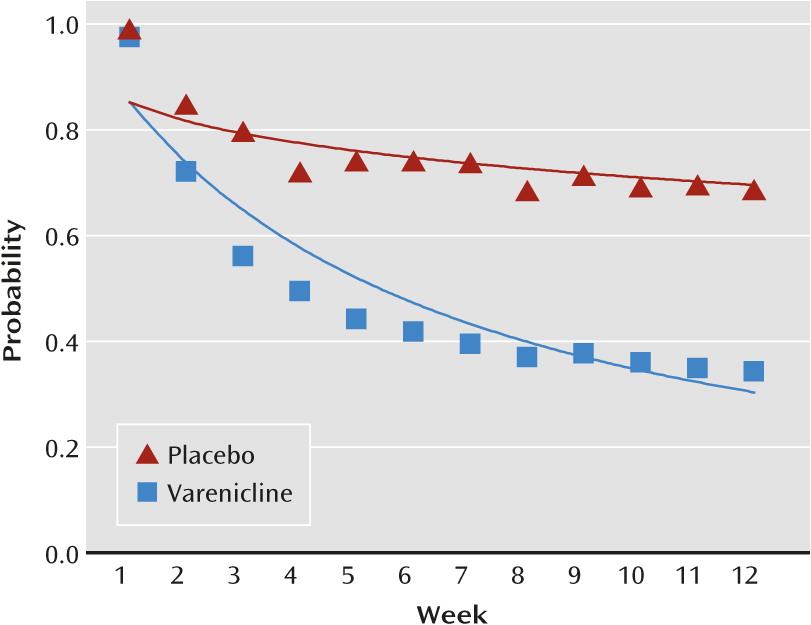
Smoking abstinence was significantly greater for patients treated with varenicline relative to those who received placebo (p<0.0001) or bupropion (p<0.0001). After 12 weeks, the estimated probability of abstinence was 30% for the placebo group and 68% for the varenicline group (relative risk=2.24, 95% CI=2.21–2.27) (
Figure 1). There was no evidence of a treatment-by-psychiatric illness interaction, indicating that varenicline is comparably effective in patients with and without a current psychiatric disorder or a history of psychiatric illness. In the three studies that included bupropion, the probability of abstinence at 12 weeks was greater for the varenicline group (78%) relative to the bupropion group (64%) (relative risk=1.22, 95% CI=1.16–1.29) (
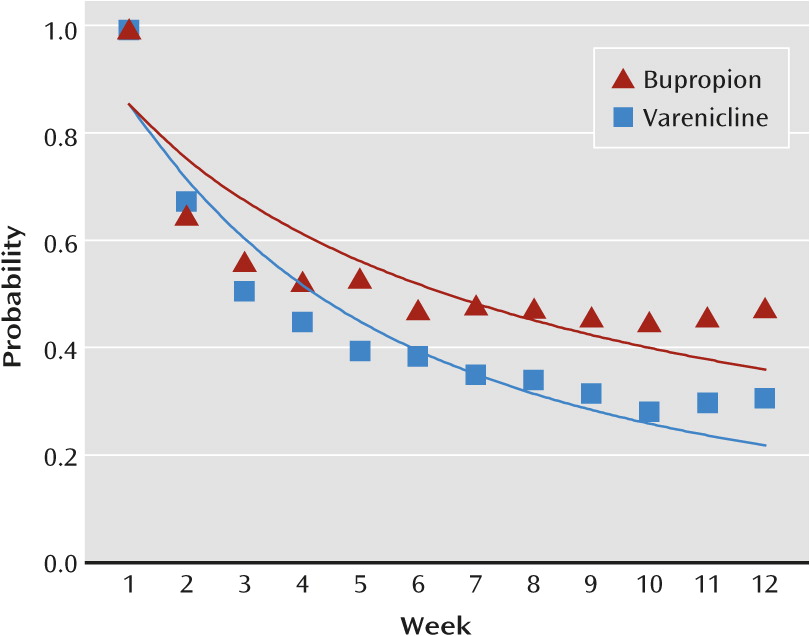
Figure 2).
Observational Study
The unadjusted overall rates of neuropsychiatric adverse events were 2.38% for varenicline and 3.17% for nicotine patch (p<0.0001). After propensity score matching, the rates were 2.28% for varenicline and 3.16% for nicotine patch (odds ratio=0.72, 95% CI=0.62–0.83, p<0.0001). Decreases in drug-induced mental disorders (odds ratio=0.10, 95% CI=0.04–0.24, p<0.0001) and other psychiatric disorders (odds ratio=0.20, 95% CI=0.04–0.91, p<0.04) were also found (
Table 3). The only disorder more frequently observed in varenicline-treated patients was transient mental disorder; however, there were few such events (nine cases for varenicline [0.05%] and four for nicotine patch [0.03%]), and the results were not statistically significant (0.06% for varenicline and 0.02% for nicotine patch after propensity score matching). In general, the results with and without propensity score matching were similar. When patients with a neuropsychiatric event (i.e., a disorder or a suicide attempt) in the previous year were excluded, the results were again similar. The unadjusted overall rates of neuropsychiatric adverse events were 0.53% for varenicline and 1.13% for nicotine patch (p<0.0001). After propensity score matching, the rates were 0.52% for varenicline and 1.09% for nicotine patch (odds ratio=0.47, 95% CI=0.35–0.65, p<0.0001).
Discussion
Analysis of longitudinal neuropsychiatric event data across the 17 randomized placebo-controlled trials of varenicline revealed no evidence of an increased risk of suicidal ideation or behavior or of neuropsychiatric events (depression, aggression, and agitation potentially associated with a greater risk of suicidal behavior) with varenicline. Moreover, we did not detect an increased risk of depression-related events after treatment discontinuation. There is, however, clear evidence that varenicline can cause side effects such as nausea, illustrating our ability to detect adverse events when present. A further strength of our research synthesis of the randomized controlled trial data is that we included two new studies, conducted with patients with current diagnoses of depression or schizophrenia, and identified additional study subjects with a history of psychiatric illness from the other studies. Overall, 13% of the sample had a current or past psychiatric illness, allowing us to determine the extent to which current or past psychiatric illness moderated the effect of the drug on both efficacy and safety. No evidence of a moderator effect was found, indicating that patients with psychiatric illness (current or past) are not at increased risk of developing neuropsychiatric events when treated with varenicline.
In terms of efficacy, 12 weeks of treatment with varenicline produced a 124% increase in the rate of abstinence relative to placebo and 22% relative to bupropion. The number needed to treat based on the estimated abstinence rates at 12 weeks was 2.63. A similar conclusion was reached by the recent Cochrane Collaboration review of nicotine receptor partial agonists for smoking cessation (
2). The researchers found that the chances of successful long-term smoking cessation were two to three times better for varenicline compared with pharmacologically unassisted smoking cessation attempts and that the main adverse event was nausea at mild to moderate levels, which tended to subside over time. With respect to neuropsychiatric events, the researchers concluded that “there is little evidence from controlled studies of any link between varenicline and psychiatric adverse events.” We reach the same conclusion based on a more complete analysis of the data. The DOD data showed that eight of the nine neuropsychiatric event types examined were less frequent in patients using varenicline than in those using nicotine patch. The only disorder more frequently observed in varenicline-treated patients was transient mental disorder; however, there were few of these events (0.05% for varenicline and 0.03% for nicotine patch), and the apparent difference was not statistically significant. Similar results were obtained when patients with a past-year psychiatric illness were included or excluded. These results are similar to recent FDA (
12) and DOD (
13) reports regarding the absence of risk for psychiatric hospitalizations in VA and DOD patients treated with varenicline relative to those treated with nicotine patch. We note that the original DOD investigators (
13) focused on inpatient events because outpatient events “are more likely to suffer from misclassification and should be interpreted with caution” and that “in outpatient settings, codes may represent instances of routine psychiatric care rather than adverse neuropsychiatric events.” While we agree that the absence of an observed effect for neuropsychiatric hospitalizations is a critically important finding and stands on its own, our analysis of the combination of inpatient and outpatient events adds further to the finding by ruling out the possibility that important events such as suicide attempts that did not lead to hospitalization would be overlooked in such an analysis. Furthermore, we do not see why the concern that outpatient treatment may be for a psychiatric illness unrelated to the medication should not apply equally to inpatient treatment, and we believe that the outpatient reports of neuropsychiatric events provide comparably meaningful and useful data, with the advantage of greatly expanding the sample size and number of outcome events.
It is possible that the decreased risk of neuropsychiatric events seen in the outpatient data for varenicline relative to nicotine replacement therapy resulted from clinicians prescribing nicotine replacement therapy instead of varenicline for patients at higher risk of such events. This explanation is tempered by the fact that the DOD data were for a period before the FDA warning.
The other large-scale observational study, conducted by Gunnell et al. (
8), also failed to identify any significant difference between varenicline and nicotine replacement therapy in rates of depression and suicidal events. These observational studies are important because they demonstrate that the results of the randomized controlled trials generalize to the clinical population of patients treated with smoking cessation products and are not limited by the inclusion and exclusion criteria of the specific randomized controlled trials.
Signals have been identified, however, in spontaneous reports of depression and suicidal events made to the FDA’s Adverse Event Reporting System/MedWatch program. As previously noted, a possible safety signal for varenicline (relative to bupropion and nicotine replacement therapy) has been reported (
5) for depression and suicidal events. As noted by Cahill et al. (
2), however, the limitations of this class of data include “confounding by indication, underreporting, double-counting from multiple sources, and lack of representativeness, which limits generalizability” (
4). They also note that because of heightened media coverage and FDA warnings, neuropsychiatric events may be more likely to be reported to the FDA for varenicline than for nicotine replacement therapy. The FDA also cautioned (
17) against drawing causal inferences from such data, noting that there is often insufficient information on the report forms to evaluate the event and that not all adverse events are reported to them; hence they “cannot be used to calculate the incidence of an adverse event … in the U.S. population.”
In an effort to shed further light on this question, we obtained the same FDA data analyzed by Moore et al. (
5) and followed their methodology for including and excluding adverse event reports. We were able to obtain a reasonably similar total number of events for varenicline: 10,291, compared with 9,575 reported by Moore et al. for the period 1998–2010. Note that this time frame includes 8 years before varenicline’s entry into the market and extends through the period of media attention and FDA warnings. In our analysis, we restricted the time frame to the period during which varenicline was on the market and before the media attention and FDA warnings, which could have stimulated reporting (the second quarter of 2006 through the third quarter of 2007). We found no evidence of a safety signal for varenicline relative to nicotine replacement therapy during this period for suicidal events (odds ratio=0.69, 95% CI=0.40–1.26), an effect for depression events (odds ratio=1.72, 95% CI=1.08–2.84), and no effect for aggression events (odds ratio=0.58, 95% CI=0.27–1.37). By contrast, significant effects of a much greater magnitude for bupropion relative to nicotine replacement therapy were observed during this same period (suicidal events: odds ratio=6.85, 95% CI=3.18–14.77; depression events: odds ratio=5.17, 95% CI=2.44–10.82; aggression events: odds ratio=7.64, 95% CI=2.90–20.58). Given the limitations of these data, their interpretation remains difficult; however, these results at least offer the alternative explanation that the safety signals identified for varenicline regarding neuropsychiatric events may have been produced by stimulated reporting.
In summary, our synthesis and reanalysis of all sponsor-conducted randomized placebo-controlled trials and an original analysis of a large DOD observational data set revealed no evidence that varenicline is associated with adverse neuropsychiatric events and confirmed nausea as a side effect. These findings are generally consistent with the literature and other randomized controlled trials and large observational studies. Our analysis reveals evidence in support of the superior efficacy of varenicline relative to both placebo and bupropion, thus indicating considerable benefit without evidence of risk of serious neuropsychiatric adverse events. These findings generalize to patients with and without a recent history of a psychiatric disorder.