Circannual rhythms of behavior are commonly observed among animals exposed to seasonal variations in temperature and daylight (
6). Seasonal influences on the mood and behavior of humans, however, were largely ignored through most of the 20th century (
7). In 1980 it was reported that bright light could suppress pineal gland melatonin production in humans (
8), as earlier described in animals, and in 1984 winter seasonal depression (
9), also known as seasonal affective disorder (SAD), was described. Winter SAD is characterized by seasonal experience of sadness, interpersonal difficulties, and physical symptoms including decreased activity, increased sleep time, carbohydrate craving, increased appetite, and weight gain. These symptoms usually respond to bright light therapy (
9). A wide range of studies have demonstrated the efficacy of light treatment for SAD and have minimized the possibility that light treatment works by a placebo effect (
10–
12). Moreover, it has become clear that mild winter seasonal diminishment of mood and energy is common, even in people who do not qualify for a diagnosis of major depression (
13). With these findings, the common link of light responsiveness between humans and animals could no longer be ignored.
A scientific mystery, however, was created. Researchers were left in the dark concerning the molecular mechanism by which humans absorb the light that has energizing and antidepressant effects. The First Law of Photochemistry, first articulated almost two centuries ago, postulates that light can produce a chemical reaction only by being absorbed by a molecule (
14,
15). This fundamental law of nature means that for light to have a chemical effect, a molecule must directly mediate that effect. In the absence of knowing what molecule or molecules are being directly acted on by light, the physiology of light treatment of winter depression remains a black box.
In this review we will consider possible candidates for mediators of light effects in winter SAD, beginning with well-known light-absorbing molecules, and will consider novel evidence supporting more recently studied molecular mechanisms. Identification of the relevant photoreceptor or photoreceptors would truly open a window into the brain and the chronobiological physiology it regulates.
Photoreceptors in the Eye
Soon after the description and classification of SAD, a controlled trial compared the therapeutic effects of eye versus skin light treatment and provided evidence that light’s therapeutic effects in SAD were likely mediated through the eyes (
16). These results were consistent with a wide body of literature establishing that light’s effects on the suprachiasmatic nuclei (the core mammalian biological clocks in the brain) are mediated through the eyes (
17,
18).
Assuming that light’s effects were mediated through recognized photoreceptors, researchers initially hypothesized that the known visual photoreceptors, rod and/or cone rhodopsin-based molecules, were the primary receptors for circadian regulation and presumably for winter depression treatment as well (
19–
22).
The classical human photoreceptors are the rods and cones that permit vision through the eyes. Distributed in a layer throughout the back of the retina, rods and cones are precisely arrayed cells containing layers of various opsin molecules, which are specialized light absorbers that convert light energy into neural signals that are integrated in the brain to produce vision. Rod cells are sensitive to very dim light, permitting colorless vision in the night and near darkness. Cone cells are sensitive to brighter light, permitting color vision during the daytime and in bright light. Opsins are better able to absorb some specific wavelengths (or colors) of light than others: the three cone opsins in humans and primates absorb best in the red, green, and blue wavelengths of the spectrum. The integration of the neural signals from the different cones permits what humans sense as full color vision. As rod and cone opsins were the historically known photoreceptors in the eye, and as they had direct neural connections to the brain, it was logical to think that these also served as the primary mediators of light’s effects on the brain in winter SAD.
With a presumed ophthalmic pathway for light’s effects, it also became logical to consider that any disturbance predisposing to SAD could be found somewhere along the physiological pathways from eye to brain. Results of such investigations on the neurological front end of the visual system in SAD have been inconsistent, varying from no finding of significant visual processing abnormalities to depressive-state-related subsensitivities to environmental light (
23–
25). The molecular mechanism of visual process dysfunction in SAD has remained unclear, although recent work by Roecklein and colleagues (
26) may be explanatory in a small percentage of people with the disorder (as discussed in the following section).
Using commonly accepted principles of photobiology, identification of putative photoreceptors is classically done by matching the wavelengths of light best able to have a desired effect with the wavelengths of light best absorbed by a candidate light-absorbing molecule (called a chromophore). If a molecule absorbs light best at the exact wavelengths at which the desired effect is seen, almost like a hand fitting in a glove, scientists consider this strong supportive evidence that the molecule under consideration is mediating the studied effect and is a relevant photoreceptor (
27).
The first study that directly attempted to identify the photoreceptor(s) in SAD compared equal photon densities of blue versus red versus white light treatment for the disorder (
28). Treatments were administered as white light containing all visible wavelengths (2,236 photopic lux), predominantly red light (603 lux), or predominantly blue light (638 lux). (Lux is a measure of how bright light appears to the human observer. Middle-wavelength or yellow to green photons appear brighter than long-wavelength red photons or short-wavelength blue photons.)
If stimulation of red or blue cone photoreceptor molecules mediating vision were mediating the antidepressant effects of light, the corresponding color of light should have proven effective. The failure of red or blue light to be effective in comparison to white light suggested to the researchers that the response in SAD was mediated by green-sensitive cones, rods (which also are maximally sensitive to green wavelengths), some combination of rods and cones, or a still unknown photoreceptor. Green light was then assumed to be likely to have antidepressant properties. A follow-up study compared green light (4,680 lux) and red light (603 lux). Photon densities were the same as those used in the prior study. Green light had a significantly better therapeutic effect than red light and appeared comparable in efficacy to the previously studied white light (
29). These studies were consistent with the hypothesis that green light was effective, or most effective, in the treatment of SAD. In both of these studies, however, the brighter-appearing light, that is, the light with higher intensity in lux, was more effective, so these results are consistent with the alternative hypothesis that total apparent brightness (lux) determined therapeutic effect. In a third study of green light (2,367 lux) versus white light (1,103 lux), both proved therapeutic, and thus the results could not distinguish between these hypotheses. Together, these studies did suggest that the most effective wavelengths used in light treatment corresponded with those wavelengths that appeared brightest, i.e., were the best absorbed by the rods and cones for vision.
Recent data have shown that mice, which are nocturnally active, also have ultraviolet-sensitive (UV-sensitive) cones in the retina that play a major role in regulating their circadian physiology and sleep (
30). Whether such cones exist in humans and whether they are applicable to SAD is still unknown. More broadly, the applicability of behavioral models based on nocturnally active rodents to diurnally active humans may have significant limits due to critical differences between species in the biology, physiology, and by definition, behavioral patterns and responses to light (
31–
33). Some controlled trials in SAD have explored whether UV light is necessary for treatment of SAD. These studies indicated that although UV wavelengths may or may not have antidepressant effects, UV light is not required for the treatment response and would therefore be contraindicated because of possible risks of stimulating skin cancers or ocular cataracts (
29,
34–
36).
Melanopsin
Much of the research into affective disorders and chronobiology grew out of a model linking advancement of the phase (timing) of the biological clock to antidepressant effects (
37). Researchers made the assumption that the photoreceptors that mediate the antidepressant effects of light in SAD are the same as those that shift circadian rhythms in humans. As some evidence suggests that the antidepressant effect of light in SAD is effective in producing a phase advance (
38,
39), the assumption is parsimonious and tenable. Circadian phase shifting has not been shown to be a requirement for light’s efficacy (
39), however, and the assumption of identical photoreceptors for antidepressant and phase-shifting effects remains unproven.
Concurrent animal research further led to acknowledgment that rods and cones might not be the only critical photoreceptors for circadian rhythms (
40). Following a report showing that hamster circadian rhythm photoreceptors exhibited properties that were unusual for rod and cone opsins (namely, the capacity for integration of time and intensity and a high threshold for bleaching) (
41), serious consideration was given to the possibility that other molecules might mediate these effects. This view was supported in humans by the finding that exposing the eyes to 6,000 lux of white light could suppress melatonin production in some humans with complete visual blindness (
42).
During the 1990s, work with mice with hereditary retinal degeneration, such as the
rd/rd mouse, indicated that some novel photoreceptor must be involved in circadian entrainment. The
rd/rd mice had no rods and few cones, had no perceptual sensitivity to light, but had fully preserved circadian entrainment (
43). Some studies suggested the importance of a novel photoreceptive system with maximal sensitivity in the blue wavelengths; for instance, the maximum sensitivity for circadian phase shifting was near 480 nm in retinally degenerate mice, while the maximal sensitivity in normal mice was nearer 500 nm (
44). Work in the transgenic
rd/rd cl mouse, which has no cones or rods at all, eventually established that some novel non-rod, non-cone system must have the capability of driving the circadian rhythm system in rodents; presumably this would be true for humans also (
45,
46).
Years of work bore fruit with the identification of a new human opsin, melanopsin, found in the cells of the mammalian inner retina (47). Multiple lines of evidence later established that melanopsin is the photoactive pigment in a subset of directly photosensitive ganglion cells that have a peak sensitivity near 480 nm and project to the suprachiasmatic nuclei (48). These ganglion cells exist in primates and also receive input from classical photoreceptors, receiving excitatory input from rods and middle- and long-wavelength cones and inhibitory input from short-wavelength cones, as well as being activated intrinsically by a melanopsin-based mechanism. These cells appear to drive the human pupillary light reflex: by using conditions that elicit the melanopsin component of pupil response, a putative melanopsin action spectrum in humans has been determined that is consistent with an opsin absorption curve with a maximum around 482 nm (49). Further research has demonstrated that the melanopsin system has a strong effect on the circadian system. This research has also shown the complexity of the effect of light on the brain. Knockout
Opn4 −/− mice, which lack melanopsin, have altered circadian responses but are able to be entrained to light, implying that rods and cones do have access to the central clock as well (
50,
51).
The discovery of melanopsin, whose absorption spectrum peaks in blue wavelengths, and subsequent work showing that blue (446–480 nm) wavelengths of light were particularly effective for suppression of melatonin production in humans (
52,
53) led to renewed interest in blue light treatment for SAD. A number of other studies in humans have suggested that short-wavelength blue light is optimally effective for various circadian functions, including phase shifting (
54,
55) and alertness induction (
56). Two studies using light-emitting diodes studied the therapeutic effect of blue or blue-weighted light in SAD. In one study, narrow-band blue light-emitting diodes (398 lux) were convincingly more effective than dim narrow-band red light (23 lux) (
57). A second study looked at the effect of 1,350-lux white light that was weighted toward the shorter-wavelength end of the spectrum, i.e., the energy distribution of the emitted light was characterized by having a main spectral emission peak at approximately 464 nm but also had a broader, secondary spectral peak near 564 nm (
58). Of the energy emitted over the visible range of 400 to 700 nm, about 48% was emitted over the range 420 to 508 nm, and 37% was emitted over the range 512 to 616 nm. Collectively, the emitted light appeared bluish-white. Both studies obtained therapeutic effects similar to those obtained in studies using typical 10,000-lux broadband white light. A separate recent study showed no difference between “blue-enriched” and white light and found no superiority of blue over white light, although both conditions may have produced a maximal treatment response (
59). Human data suggesting that green-wavelength light (approximately 500 nm) can be highly effective in shifting rhythms and in suppressing melatonin emphasizes that middle-wavelength light does access the circadian rhythm system (
60). As already noted, green light does appear to have a potential therapeutic effect in SAD, whether through stimulation of the melanopsin system or through classical rod and cone photoreceptors. At this time, the most effective wavelengths of light for treatment of SAD remain unclear.
Melanopsin researchers then began to consider specifically whether melanopsin might play a role in mediating light’s effects in SAD. Hypothesizing that variations in melanopsin candidate genes might affect light input to the brain and increase vulnerability to SAD, one study explored haplotypes of the melanopsin gene, including specific single nucleotide polymorphisms that result in coding variants of the melanopsin protein (
61). A single missense variant (P10L) was associated with increased risk of SAD, specifically for individuals homozygous for the minor T allele when compared to other genotypes (C/T and C/C). This variant was seen in 5% of individuals with SAD. Further work by the Pittsburgh research team has indicated a modest mean decrease in the post blue light illumination pupillary reflex regulated by melanopsin in a small group with SAD (
26). It would be important to discover how light treatment mediates the antidepressant and energizing effect of light in these people.
Humoral Phototransduction
But what of the 95% of people with SAD for whom an abnormality in melanopsin phototransduction does not explain their vulnerability to seasonal change? Do most SAD patients have normal melanopsin-mediated light absorption but abnormal processing of the melanopsin-mediated light signal beyond the melanopsin molecule? Might there be a different photoreceptor that mediates seasonal or antidepressant effects of light in addition to the circadian effects of light that are mediated by melanopsin, and to some degree rods and cones?
While Darwin reported in 1880 that “hardly anyone supposes that there is any real analogy between the sleep of animals and that of plants” (
62), seasonal and circadian behaviors of plants have been observed for millennia.
The responses of many biological rhythms, including sleep, to manipulations of ambient light in animals strikingly resemble responses in plants. If one takes a flight across several time zones, the commonly recognized phenomenon of jet lag will occur, in large part manifested by awakening and sleeping at undesired times of day in the new environment. Several days may be required before one adapts to the new time zone. Similarly, if one could take a morning glory plant—which typically blooms every morning and closes its petals for the night—on the same flight, for the same reasons it would develop its own form of jet lag, in large part manifested by blooming at the “wrong” time of day in its new environment. Such jet lag phenomena in plants and animals, in the laboratory and in the field, can be created and treated by properly timed exposure to bright light and darkness. Considering that the daily act of “engaging the environment,” whether by a human’s awakening or by a morning glory opening its petals to the sun, is the most fundamental form of behavior, seen in virtually every plant and animal species, we can ask whether molecular mechanisms of chronobiological light absorption might be conserved across the plant and animal kingdoms.
Drawing specifically on this evolutionary argument that seasonal and circadian influences of light have significant behavioral similarities in plants and animals, a model of “humoral phototransduction” was proposed (
63). This model argued that the well-known tetrapyrrole-based light-absorbing molecules that regulate plant energy and chronobiology might have analogues in animal biology and, in particular, proposed that blood-borne tetrapyrroles directly mediate light’s effects in SAD through the eyes. In plants these molecules are chlorophyll, containing a closed-ring tetrapyrrole, and phytochrome, an open-ring tetrapyrrole. Synthesized along identical molecular structural pathways in plant and animal species until the final chemical reactions that make them distinct, the light-absorbing chromophore structures of chlorophyll and phytochrome are analogous to the light-absorbing chromophores of hemoglobin and its breakdown products of biliverdin and bilirubin. As the retina is highly vascular with blood vessels unshielded by protecting skin or tissue, the eye is the site of the most efficient exposure of blood to light. In this model, the pigments mediating the effects of light include the plant chlorophyll analogue hemoglobin, the phytochrome analogues biliverdin and bilirubin, and hemoproteins, such as heme oxygenase and nitric oxide synthase.
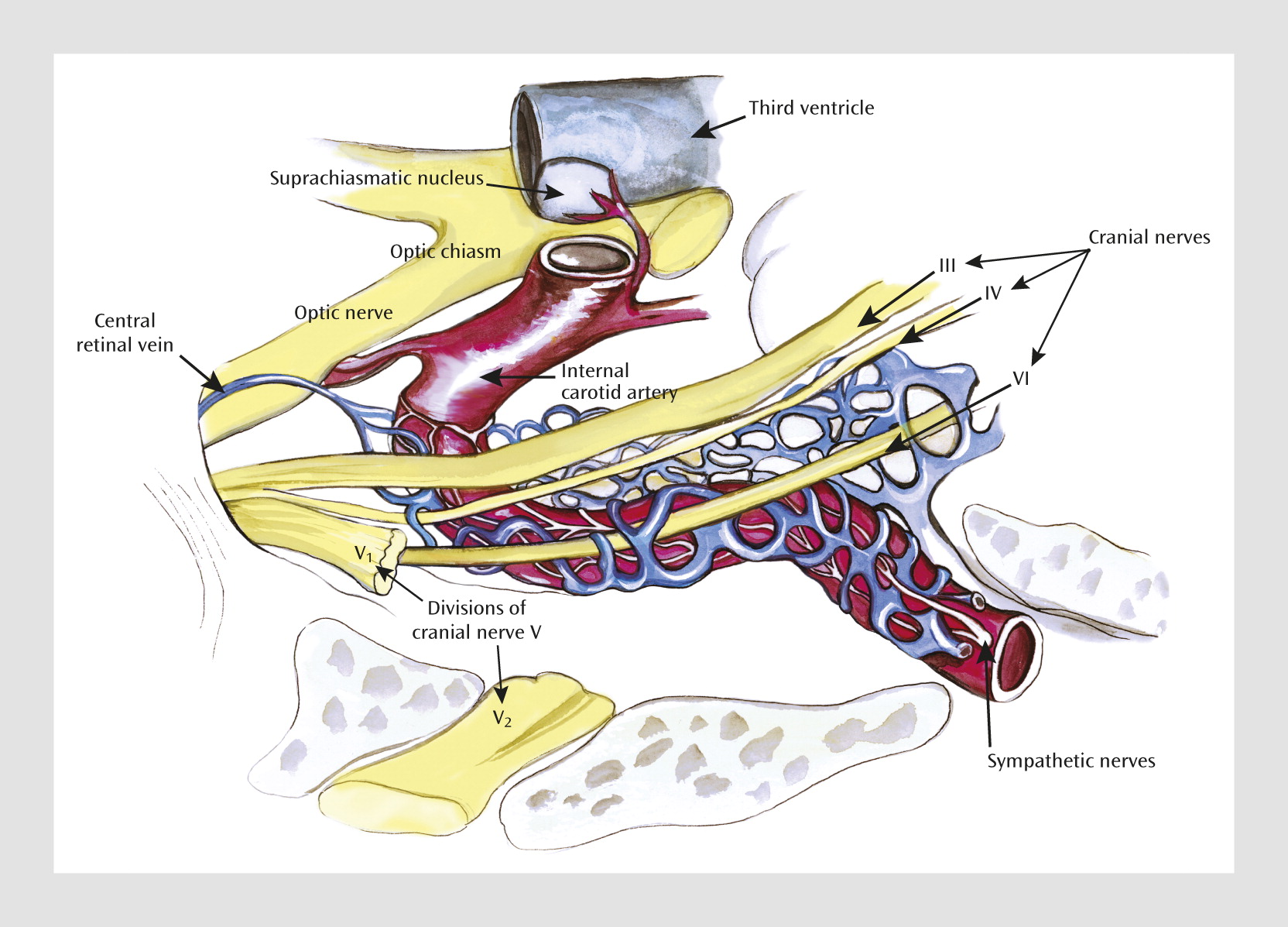
A novel and direct prediction of this “humoral phototransduction” model is that light will stimulate the release by hemoglobin and production by hemoproteins of what today are described as the “gasotransmitters” carbon monoxide (CO) and nitric oxide (NO) in retinal venous blood. These messengers could then drain to the cavernous venous sinus plexus. The cavernous venous sinus plexus has long been considered a remarkable structure of multiple blood vessels enwrapping the internal carotid artery (
Figure 1). These gaseous transmitters could then diffuse into the internal carotid artery and provide a humoral signal of daylight to the adjacent suprachiasmatic nuclei. Countercurrent mechanisms for local chemical regulation have been demonstrated in diverse fish and animal physiological systems for decades. A countercurrent transfer of reproductive hormones from venous to arterial blood has indeed been demonstrated in the cavernous sinus (
64).
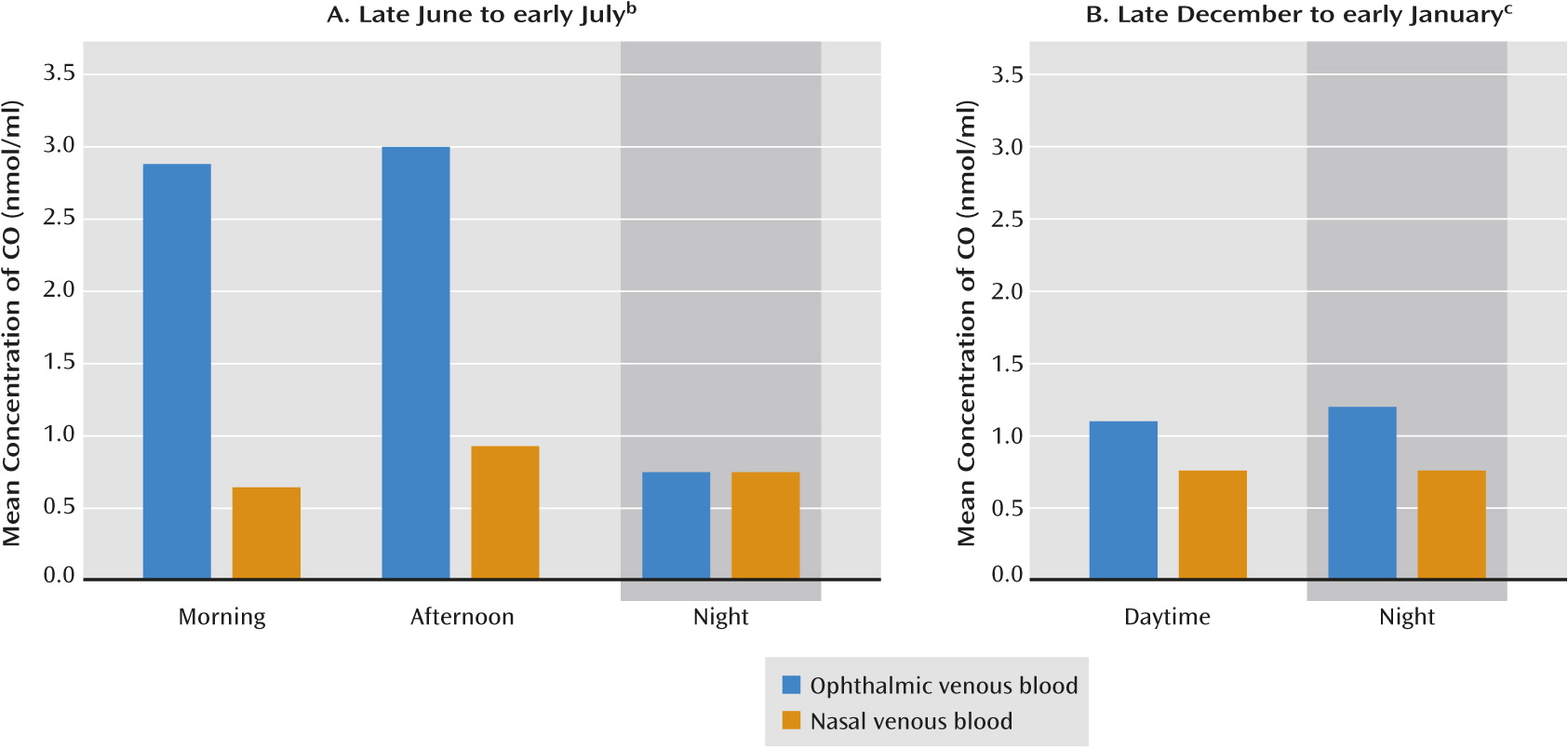
A key building block of this model was recently confirmed by Koziorowski and colleagues, who examined seasonal and diurnal variation in venous blood CO from the eye in a male wild boar-pig crossbreed (
65). This animal model is particularly useful for study both because of the large seasonal variation in the animal’s behavior and because of the accessibility of its relatively large major eye and brain blood vessels, which are generally similar in structure to those of humans. Koziorowski et al. compared CO levels in ophthalmic and nasal venous blood in the morning, afternoon, and night in eight animals during the longest days of the year and eight animals during the shortest days of the year at 50°N latitude.
During the long-photoperiod days at the time of the summer solstice, the concentration of CO in ophthalmic venous blood (draining from the eye) was more than three times that found in nasal venous blood (not draining from the eye) (p<0.05) (Figure 2A) and more than twice that of CO concentrations in systemic arterial and venous blood. Only a small difference between ophthalmic and nasal venous blood was seen during the dim, short-photoperiod days near the winter solstice (p<0.05), with the concentration of CO in ophthalmic venous blood in winter approximately one-third the concentration in summer (p<0.05) (Figure 2B). The researchers concluded the CO is released from the eye into ophthalmic venous blood according to the intensity of sunlight. Those results are consistent with the humoral transduction model, which would then predict that the CO released diffuses via the cavernous sinus into the arterial blood supply of the brain.
There are at least two plausible (and complementary) mechanisms for the observation of elevated CO seen in retinal venous blood after bright summer light. First would be the photodissociation of CO bound to heme iron groups in a reaction that was discovered in the 19th century by Haldane and Lorrain Smith (
66). This process was dismissed as clinically irrelevant by its discoverers and long ignored by biologists owing to the mistaken belief that CO was biologically inert, except as a poison that interfered with hemoglobin’s normal oxygen-carrying capacity. A second mechanism would be the well-documented stimulation by bright light of heme oxygenase, the enzyme that cleaves heme moieties to form CO and biliverdin (
67). This photostimulation simultaneously dissociates bound CO from the heme moiety of heme oxygenase and activates the enzyme itself to produce more CO.
Biliverdin is immediately converted in mammals to bilirubin by biliverdin reductase. Along with melatonin (
68), biliverdin and bilirubin are among nature’s most potent antioxidants (
69). Bilirubin is a photoactive molecule with daily and seasonal variation, rising at night and declining in the day, higher during the months when the days shorten and lower during the months when the days lengthen (
70,
71). Bilirubin’s daily and seasonal links have been less well studied than those of melatonin. Bilirubin thereby parallels melatonin in having circadian and circannual rhythms, antioxidant properties, and regulation by light. It might also play an important role in regulating seasonal rhythms. Qualitatively, it exhibits a circadian signal that is different from that of melatonin. Whereas peripheral melatonin levels regularly begin to rise around dusk, peak in the middle of the night, and decline to baseline by dawn (
72), peripheral bilirubin levels climb during the night and peak near dawn, then decline during the daylight to reach their minima near dusk (
70). In contrast to melatonin, bilirubin might provide a distinct and complementary humoral signal of environmental light and internal time to the body.
Preliminary data supporting a link between bilirubin and SAD was subsequently found in a study in which nocturnal bilirubin levels in patients with winter depression were shown to be significantly lower than those in age- and gender-matched normal comparison volunteers and were significantly higher after morning light treatment, commensurate with clinical improvement (
73).
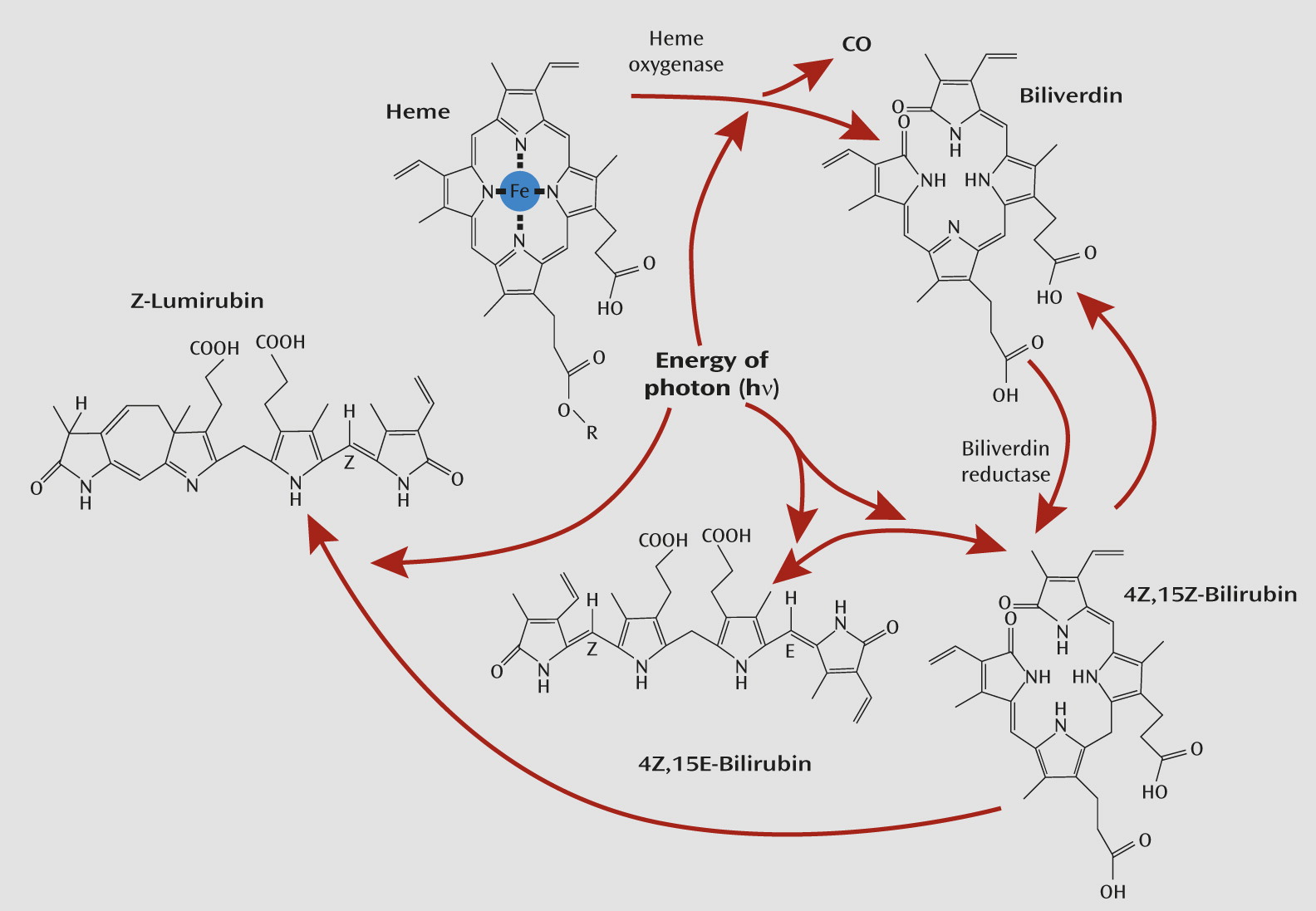
Furthermore, bilirubin is a tetrapyrrole and is itself sensitive to light. The sensitivity of hyperbilirubinemia to bright light has been known to be of therapeutic importance for neonatal jaundice for decades (
74). In the presence of sufficiently bright light, the normal 4Z,15Z-bilirubin form quickly undergoes reversible configural isomerization to form 4Z,15E-bilirubin (see
Figure 3). The optimal wavelengths for stimulating this reaction lie in the blue region of the spectrum (
74). The latter isomer is a less lipophilic form of bilirubin that is more easily excreted unchanged in bile. Bright light also can rapidly and irreversibly convert normal 4Z,15Z-bilirubin to another structural isomer: Z-lumirubin. This form is also less lipophilic than its parent bilirubin compound and can also be excreted in urine, unlike normal bilirubin, which is normally excreted only in bile (
74,
76). As such, both 4Z,15E-bilirubin and Z-lumirubin would also be less likely to have the capacity to diffuse from the cavernous sinus into the internal carotid artery and signal dark to the brain. In a pilot study, whose results correspond with this proposal, during light therapy in adults, within 10 minutes statistically significant increases in lumirubin levels were seen in urine, and within 50 minutes statistically significant decreases in bilirubin were seen in plasma (
77). Bilirubin is an attractive photoreceptor candidate for mediating some of light’s effects in SAD and in affecting circadian chronobiological rhythms. Bilirubin might therefore be both a signal and a transducer.
In short, the tetrapyrrole pigments of blood are photoactive and are photoactive at environmental levels of light exposure. Exposure of the eye to light appears to cause a physiological release of CO and may generate biliverdin, which is rapidly converted to 4Z,15Z-bilirubin. This form of bilirubin itself is converted by light to two less lipophilic isomers. Our model thus generates multiple signals of environmental light. Only empirical data will reveal the nature of the postulated tetrapyrrole signal to the brain. On the basis of the preceding data, it seems likely that retinal venous CO is a potential biomarker of circadian or seasonal rhythms and is directly affected by light’s actions on retinal blood. Nevertheless, these data by themselves do not prove that retinal venous CO or other gasotransmitters such as NO, or bilirubin, are in fact providing specific signals to the brain and regulating circadian or seasonal responses. Demonstration of changes in levels of chronobiological biomarkers regulated by the suprachiasmatic nuclei (such as peripheral melatonin levels) by the infusion of exogenous CO or NO in ophthalmic venous blood and demonstration of countercurrent transfer of CO or NO across the cavernous sinus into the internal carotid artery or increased delivery of CO or NO to the suprachiasmatic nuclei would provide direct evidence of a humoral pathway from eye to brain. Such studies are underway.
Challenges and Conclusion
The primary photoreceptors that mediate light’s seasonal effects and its antidepressant effects in SAD remain unclear: the candidates include melanopsin, the rod and cone opsins (either through their direct connection to the brain or through their input to the melanopsin system), and the tetrapyrroles of hemoproteins, biliverdin, and bilirubin as discussed here. The panoply of data suggests that light’s varied effects may be mediated through a small constellation of chromophores.
This new perspective recognizes what science has been demonstrating for most of the past century, that light is a core regulator of animal biology, as commonly accepted in plants. Such a view is tenable in the context of the conservation of molecular structure of the tetrapyrrole chromophores and their biosynthetic pathways in plants and animals (
63). This view is supported by later discoveries, many of which are still emerging, of significant commonalities of plant and animal physiology, including circadian regulation, seasonal regulation, and sensory regulation (
78). If hemoproteins and bilirubin are physiological photosensors, then the ubiquitous presence of hemoglobin and its breakdown products at the core of animal biology surely places light and photosensitive regulation of bioactive gases at the core of animal physiology as well. This new perspective echoes the wisdom of the ancients of millennia ago, who recognized light’s physiological benefits for human energy and mood (
79). For that matter, the ancients may have been correct in some respects as well in linking bile and mood.
Understanding the mechanism of light’s therapeutic effect in SAD is important not only theoretically but practically. Knowing the most effective wavelength distribution may enable more efficient and more effective treatment devices. Even if a particular wavelength is found most therapeutically potent, it may not be the optimal clinical approach. For example, short-wave blue light is potentially phototoxic to the retina, and light of longer wavelength could prove safer (
80). In terms of SAD, the research agenda suggested by the First Law of Photochemistry once called simply for matching a single color of light causing a clinical response with a single color of light that a pigment absorbs. The research agenda for understanding light’s effects on human physiology today is certainly less simple but, in photochemical language, most exciting.