Major depressive disorder is among the most disabling illnesses worldwide (
1). A substantial proportion of patients do not achieve a clinically meaningful benefit despite multiple antidepressant trials and augmentation strategies (
2,
3). Treatment-resistant major depression, defined as an insufficient response to at least two adequate antidepressant treatments, is associated with low rates of improvement with currently available antidepressant treatments (
3,
4), and an intervention for refractory cases is thus an important unmet clinical need. Treatments that exert rapid antidepressant effects are a complementary unmet need, as the usual lag time to therapeutic effect is 4–12 weeks if patients show a response (
2). Modulation of monoamine neurotransmitter systems (e.g., norepinephrine, dopamine, or serotonin) is the pharmacological mechanism underlying almost all current antidepressant agents, likely accounting for their similar efficacy and therapeutic time course (
5). Therefore, engaging novel molecular targets outside of the monoamine system will likely be required in order to engender a clinically meaningful advance in depression therapeutics (
6,
7).
Converging evidence from in vivo brain imaging studies, postmortem investigations, and gene expression studies implicates abnormalities in glutamatergic signaling in the pathophysiology of major depressive disorder (
8–
10). Ketamine—a glutamate
N-methyl-
d-aspartate (NMDA) receptor antagonist—was associated with rapid antidepressant effects in patients with major depressive disorder (including treatment-resistant major depression) in several small studies and case reports (
11–
15). Antidepressant activity was observed within hours of a single subanesthetic intravenous infusion, representing a potential paradigm shift in therapeutic approaches for major depressive disorder. Methodological limitations of prior studies, however, including small study groups and the use of a crossover design with an inert placebo control condition (
11,
12), precluded definitive conclusions regarding ketamine’s antidepressant efficacy. Identifying an appropriate control condition for testing the rapid antidepressant effects of ketamine has been particularly challenging since transient psychoactive effects associated with ketamine have the potential to undermine the integrity of the study blind.
We designed the present study to test the rapid antidepressant efficacy of ketamine in a relatively large group of subjects with treatment-resistant major depression, using an active placebo control condition (i.e., the anesthetic benzodiazepine midazolam) to optimize blinding and mitigate the influence of nonspecific factors on antidepressant outcome. We hypothesized that ketamine would be superior to midazolam in improving depressive symptoms 24 hours following a single infusion. The primary outcome at 24 hours was chosen to reflect a potential rapid antidepressant effect while avoiding any overlap with the expected transient psychoactive effects of ketamine or midazolam.
Method
Study Design and Patients
The study enrolled patients at two academic sites, Baylor College of Medicine and Icahn School of Medicine at Mount Sinai, between November 2010 and August 2012. Patients were eligible to participate if they were 21 to 80 years of age, had a primary diagnosis of major depressive disorder as assessed with the Structured Clinical Interview for DSM-IV—Patient Edition (
16), and had an inadequate response to at least three therapeutic trials of an antidepressant according to the criteria of the Antidepressant Treatment History Form (
17). The form was completed by a study psychiatrist using all available information from the patient report, information provided by a family member or caretaker, pharmacy records, and medical records. Additional study inclusion criteria included a history of at least one previous major depressive episode prior to the current episode (recurrent major depressive disorder) or the combination of a chronic major depressive episode (at least 2 years’ duration) and a score of 32 or greater on the Inventory of Depressive Symptomatology—Clinician Rated (
18) at screening and within 24 hours of infusion. Patients were excluded if they had a lifetime history of a psychotic illness or bipolar disorder, alcohol or substance abuse in the previous 2 years, unstable medical illness, serious and imminent suicidal or homicidal risk, or a score less than 27 on the Mini-Mental State Examination (
19) or if they were taking contraindicated medications. Each patient had a physical examination, routine hematologic and biochemical tests, urine toxicology measurements, and an electrocardiogram (ECG) to detect unstable medical illness or substance use.
The institutional review boards at both participating sites approved the study. After complete description of the study to the subjects, written informed consent was obtained.
Study Procedures
The study patients were free of concomitant antidepressants and other psychotropic medications for the duration of the study with the exception of a stable dose of a nonbenzodiazepine hypnotic (e.g., zolpidem, 10 mg nightly). The protocol required patients to be drug free prior to the infusion, for at least 4 weeks for patients who were taking fluoxetine and for at least 1 week for those taking other medications.
Randomly assigned in a 2:1 ratio, the patients received a single intravenous infusion of ketamine hydrochloride (0.5 mg/kg) or midazolam (0.045 mg/kg) infused over 40 minutes. Selection of midazolam—a short-acting benzodiazepine and anesthetic agent—as the control condition was based in part on pharmacokinetic characteristics similar to those of ketamine: fast onset of action and short elimination half-life (
20). We sought to provide an anesthetic agent as a control condition that would mimic ketamine in terms of the time course of nonspecific behavioral effects (e.g., sedation, disorientation). The selected midazolam dose of 0.045 mg/kg is considered equipotent to 0.5 mg/kg of ketamine (
21). The study research pharmacist prepared sealed envelopes that contained the drug identity; all other study personnel, including investigators, anesthesiologists, raters, patients, and data analysts, were masked to treatment assignment.
Following admission to a clinical research unit and an overnight fast, an indwelling catheter was placed in the antecubital vein of the nondominant arm, and pulse, blood pressure, digital pulse oximetry, and ECG monitoring were instituted, according to procedures previously described (
13,
22). A trained rater conducted symptom ratings during the infusion and for 240 minutes following the start of the infusion. Patients were discharged from the research unit 24 hours after the infusion and received outpatient evaluations 48 hours, 72 hours, and 7 days postinfusion. The patients were instructed to abstain from psychotropic medications and to abstain from substances of abuse and alcohol while at home. Nonresponders were considered patients with less than 50% improvement from baseline in the score on the Montgomery-Åsberg Depression Rating Scale (MADRS) (
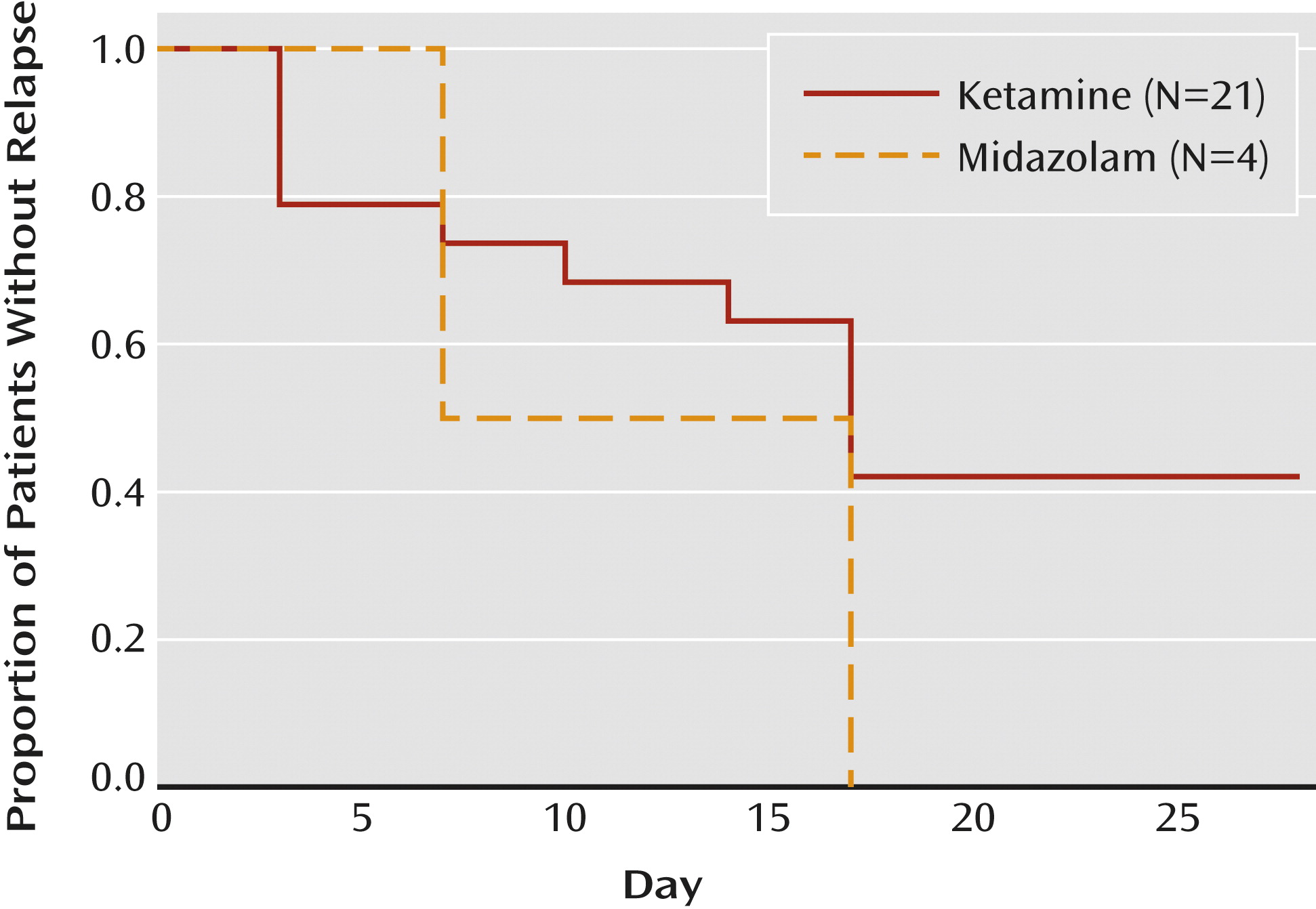
23). As directed by the protocol, we stopped following the nonresponders 7 days after the infusion. Responders were followed biweekly until relapse or for an additional 4 weeks, whichever came sooner.
Outcomes
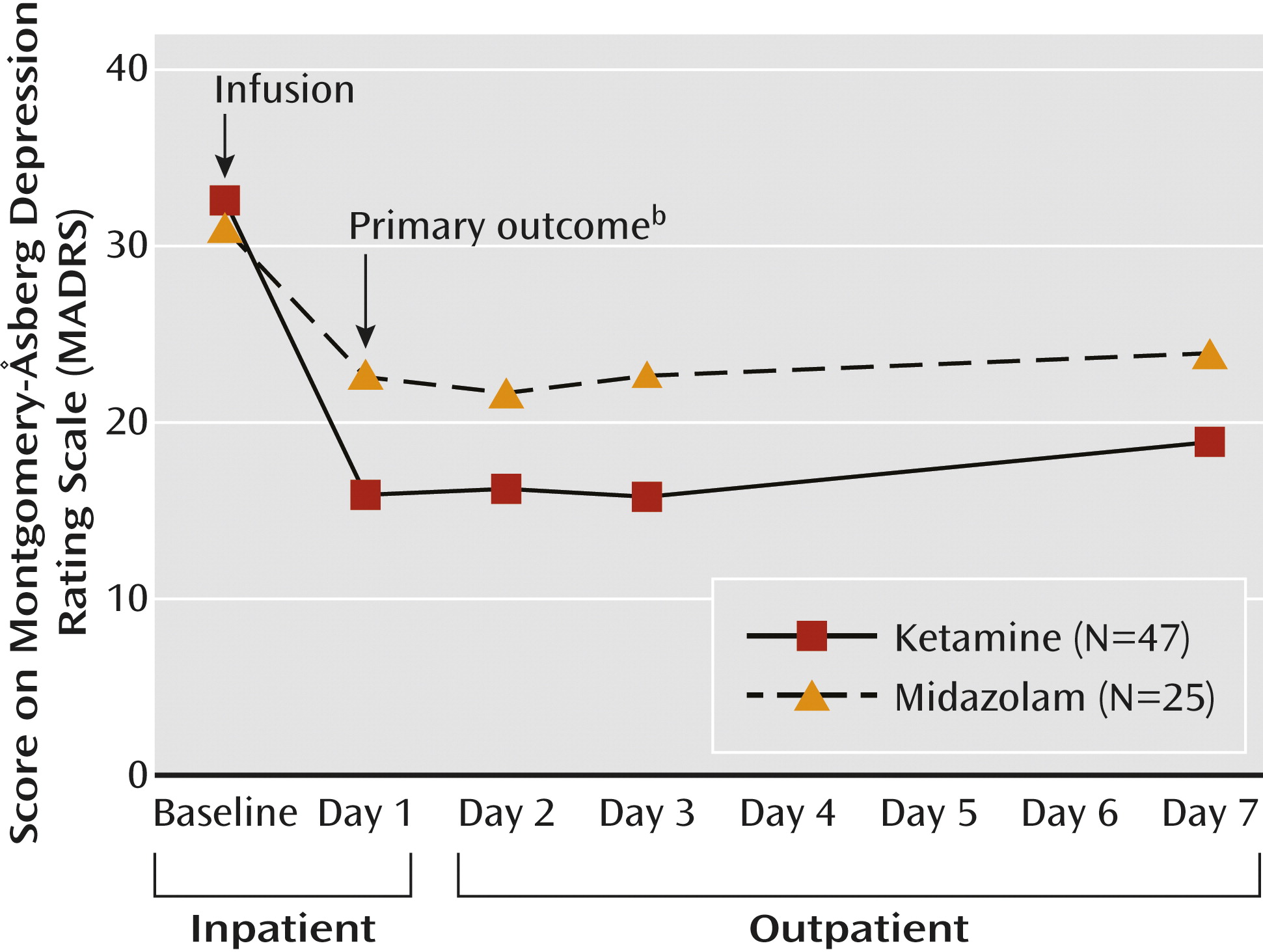
The primary outcome was reduction in depression severity as assessed on the clinician-administered MADRS (
23) 24 hours following infusion. Trained raters, who were not involved in the infusion-day procedures and who were unaware of treatment-group assignment and infusion-related side effects, performed clinical assessments for the primary outcome at 24 hours and subsequent evaluations. The raters were experienced research staff extensively trained in the use of the instruments, and the MADRS rating conventions were pilot tested in prior ketamine studies in treatment-resistant major depression (
13,
22). The two primary raters at each site achieved a high level of interrater reliability, 0.988.
Prior studies suggest that the peak antidepressant effects of ketamine occur within 24 hours of administration (
12,
13). We selected the 24-hour change in depression severity as the primary endpoint for the current study because the interval after infusion was considered long enough that acute sedating and other side effects were not likely to be contributory. The interval was sufficiently short such that the individuals who showed substantial mood improvement were unlikely to have already relapsed. Secondary outcomes included the MADRS response rate (defined as a reduction in the baseline score by 50% or more), change in score on the Quick Inventory of Depressive Symptomatology—Self-Report (
24), scores on the Clinical Global Impression (CGI) severity and improvement measures (
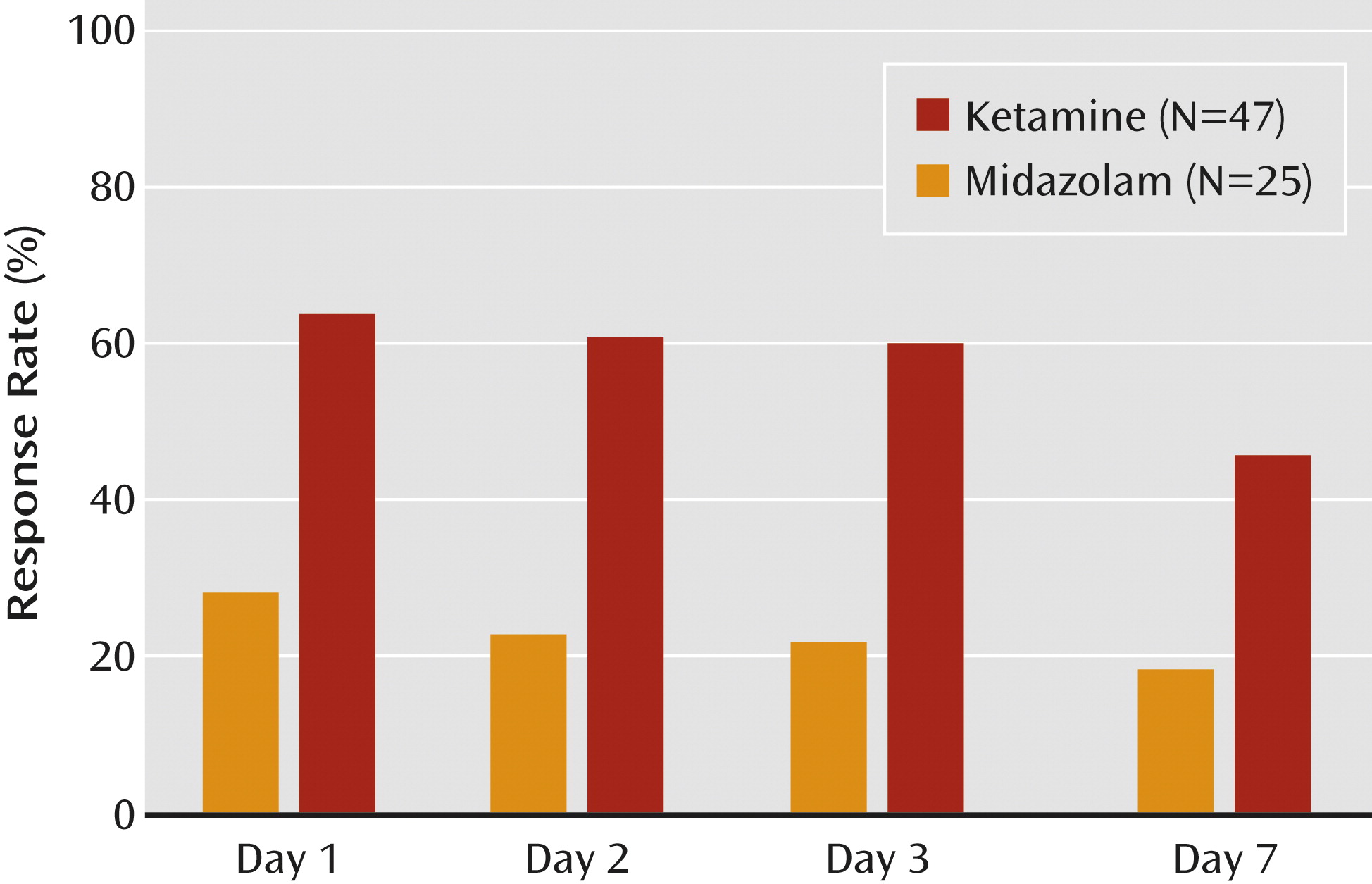
25), and durability of benefit for up to 7 days following infusion.
We recorded general adverse events, dissociative states, and psychotomimetic side effects at regular intervals throughout the study, using the Patient Rated Inventory of Side Effects (
26), the Clinician-Administered Dissociative States Scale (
27), and the Brief Psychiatric Rating Scale positive symptom subscale (
28), respectively.
Statistical Analysis
Power estimates for continuous MADRS scores and dichotomous response outcomes each assumed a two-tailed alpha level of 0.05. Conservative effect size estimates, Cohen’s d=0.71 for MADRS scores and response rates of 60% versus 15% for ketamine and midazolam, respectively, were derived from previous reports (
11,
12). A planned study group of 72 patients randomly assigned in a 2:1 ratio (ketamine versus midazolam) provided 80% and 96% power to detect a change in MADRS scores and response rates, respectively, at 24 hours as a function of treatment.
Modified intention-to-treat analyses included all randomly assigned patients with baseline measurement and at least one postbaseline measurement. Sensitivity analyses evaluated the robustness of conclusions in relation to missing data. We used general linear modeling (with the Proc Mixed function of SAS version 9.3 [SAS Institute, Cary, N.C.]) to examine MADRS scores at 24 hours as a function of treatment after controlling for baseline MADRS score and site. Exact logistic regression (Proc Logistic function, SAS version 9.3) evaluated response as a function of treatment group after adjustment for site.
Secondary analyses of scores on the Quick Inventory of Depressive Symptomatology and CGI severity and improvement scales employed general linear modeling and ordinal logistic regression (Proc Logistic, SAS), respectively, to evaluate treatment after adjustment for baseline measurements and site. Among the responders, general linear mixed modeling was used to evaluate the trajectory of change in the follow-up MADRS scores as a function of time, treatment, and the interaction of time and treatment. All statistical tests used a threshold of p≤0.05 for significance. Safety and tolerability were analyzed with the use of descriptive statistics.
Discussion
In this two-site trial in treatment-resistant patients with moderate-to-severe and persistent depressive symptoms, we found that a single low dose of ketamine, as compared with a psychoactive placebo control medication, was associated with a rapid-onset antidepressant effect. We found marked improvements in clinician-administered and patient self-report ratings of depression severity 24 hours after the ketamine infusion. The ketamine responders generally maintained the gains for several days beyond the 24-hour time point; we no longer observed statistically significant differences between treatment groups 7 days following the infusion. These data provide new support for the hypothesis that NMDA receptor modulation can accelerate clinical improvement in patients with severe and chronic forms of depression.
We consistently demonstrated the magnitude and duration of ketamine’s benefit across two sites in an ethnically and racially diverse patient group, enhancing confidence in the reliability of these findings. The large and rapid antidepressant effect of ketamine we observed in these patients with a history of three or more failed antidepressant trials is especially significant given the poor prognosis for improvement with currently available antidepressant treatments in treatment-resistant major depression (
3–
5,
29). To maximize internal and external validity, we standardized infusion-monitoring procedures and shielded the primary outcome rater from knowledge of side effects on the day of infusion. Improvements in secondary outcomes of global illness severity also supported the efficacy of ketamine in this trial.
Patients in the ketamine group experienced transient psychoactive and hemodynamic effects consistent with those in previous reports and clinical experience (
11–
15). Ketamine treatment did not increase the risk of emergent psychotic or manic symptoms over the follow-up period (see Figure SF2 in the
data supplement accompanying the online version of this article). These findings suggest that ketamine is safe in the short term for nonpsychotic depressed patients when administered at a subanesthetic dose of 0.5 mg/kg over 40 minutes. It is important to note that the safety and efficacy of ketamine in depression beyond a single infusion are largely unknown and that abuse liability and other safety concerns associated with ketamine dictate a cautious approach to its application outside of research (
30,
31). The observed hemodynamic changes in a subgroup of patients in our study encourage cardiorespiratory monitoring as an essential component of risk management.
The use of the anesthetic benzodiazepine midazolam as a control condition is a strength of the current study, although there is likely no perfect control condition for ketamine. Our objective was to select an agent that would function as a placebo (devoid of specific antidepressant effects) yet induce transient psychoactive effects designed to enhance study blinding and mitigate the nonspecific salutary impact of receiving an anesthetic agent. While the rates of general adverse events were similar across the two conditions, transient dissociative side effects immediately following study drug infusion were higher in the ketamine condition. Other agents that we considered for use as an active placebo included a sympathomimetic agent such as amphetamine. Amphetamine would have mimicked more closely the known sympathomimetic effects of ketamine (
30); however, in contrast to midazolam, amphetamine is devoid of anesthetic properties. Finally, while we considered using a true active comparator with intrinsic antidepressant properties, we found that no pharmaceutical agents were readily available that had established antidepressant properties across a time scale similar to that for ketamine. Following the establishment of the antidepressant properties of ketamine in an optimized placebo-controlled design, future studies may compare schedules of ketamine to active comparators such as electroconvulsive therapy or antidepressant-antipsychotic medication combinations.
The biological mechanisms underlying ketamine’s antidepressant activity remain largely unknown. The rapid onset of antidepressant activity we observed is consistent with preclinical work indicating that ketamine rapidly (within hours) increases the number and functioning of synaptic connections involving cortical or hippocampal neurons (
32–
34). Ketamine appears to rapidly reverse both behavioral and neuronal changes associated with chronic stress, in part through activation of the mammalian target of the rapamycin signaling pathway and stimulation of brain-derived neurotrophic factor signaling (
35). It is interesting that a recent study of patients with major depressive disorder found that carriers of the Val66Met (rs6265) single nucleotide polymorphism—representing an attenuation of BDNF functioning—had a smaller antidepressant response to ketamine (
36), in line with findings in animal models (
34,
37,
38).
Limitations of our trial include stringent enrollment criteria due to concerns about ketamine’s psychoactive effects and abuse liability. We believe that the exclusion of patients with histories of psychotic symptoms or substance or alcohol use disorders does not diminish the generalizability of our findings but, rather, offers a clinically relevant risk-mitigation strategy. A proportion of screened patients (17.2%) refused or were unable to tolerate psychotropic medication washout prior to randomization, thereby restricting participants to medication-free individuals or those able to tolerate medication washout. The efficacy of ketamine as an adjunct to ongoing therapies is a clinically relevant question not addressed in our study. Finally, we tested the efficacy of a single infusion over a brief follow-up period. The transient antidepressant response to ketamine highlights the need to identify strategies to maintain and prolong the initial response. Two studies of the glutamate-modulating drug riluzole failed to find benefit in prevention of relapse following ketamine administration (
13,
39). Additional infusions of ketamine have recently been explored to prolong the antidepressant response (
22,
40), although controlled data testing this strategy are not currently available.
In conclusion, treatment-resistant patients in a major depressive episode showed a rapid antidepressant response to a single infusion of ketamine. To our knowledge, the current study represents the largest investigation to date of ketamine in treatment-resistant major depression. Utilizing an optimized active placebo design, the trial provides new evidence for the specific antidepressant effects of ketamine, apart from its nonspecific anesthetic properties. Future research is required to test the antidepressant effects of ketamine beyond a single administration and to characterize its longer-term safety profile.