Postpartum psychosis is a psychiatric emergency that requires immediate medical attention and mental health care referral. The prevalence in the general population is estimated to be 1–2 cases per 1,000 childbirths (
1,
2). In the majority of cases, the onset is rapid and occurs within 2 weeks of delivery. Early symptoms often include insomnia, mood fluctuation, and obsessive concerns regarding the newborn, followed by more severe symptoms, such as delusions, hallucinations, and disorganized behavior. Severe mood symptoms, such as mania, depression, or a mixed state are a hallmark of the illness. Because clinical presentation, family history, and the longitudinal illness course overlap markedly with those of bipolar disorder, postpartum psychosis is generally considered a bipolar spectrum illness and not a primary psychotic disorder (
3–
5).
The initial clinical evaluation for postpartum psychosis requires a thorough medical and psychiatric history, physical and neurological examinations, and comprehensive laboratory analysis to exclude organic causes for acute psychosis. The differential diagnosis should include infectious diseases (e.g., mastitis, endometritis), eclampsia, postpartum thyroiditis (
6), and, less frequently, autoimmune encephalitis (
7,
8), primary hypoparathyroidism (
9), vitamin deficiency, stroke, and drug-induced psychosis (
10). Case reports have documented misdiagnosis of postpartum psychosis revealing a late-onset urea cycle disorder (
11) and type I citrullinemia (
12). Clinicians must ensure the adequate safety of the patient and her children, as postpartum psychosis is associated with a highly elevated risk of suicide and infanticide.
Because of the severity of the symptoms and the unpredictable nature of the illness, pharmacological treatment is typically initiated immediately. Unfortunately, however, few standardized treatment recommendations are currently available for postpartum psychosis, as research has been limited and no randomized trials have been conducted. The effects of lithium (
13,
14), antipsychotics (
15), ECT (
13,
14), estrogen (
16–
18), progesterone (
19), and propranolol (
20) have been examined. A total of 21 treatment studies of postpartum psychosis can be found in the literature of the past few decades, all of them based on small samples; the majority involve case reports, and few studies have included more than 10 patients (
21,
22).
In the absence of formal guidelines, treatment in clinical practice is typically based on the most prominent symptom dimensions. Benzodiazepines are used for insomnia and agitation, antipsychotics and mood stabilizers for psychotic and manic symptoms, and antidepressants for depressive symptoms. ECT has been described as an effective treatment option in patients with severe catatonic features. Recently, ECT has even been proposed as a first-line treatment option (
23–
25).
We have developed a four-step standardized treatment algorithm with guidance from the extensive literature on bipolar patients, and here we examine prospectively how this treatment approach might affect outcomes. The first step in treatment involves benzodiazepines at bedtime for 3 days (step 1). The purpose of starting with an initial period of benzodiazepine monotherapy is to evaluate whether restoration of sleep results in clinical remission of manic and psychotic symptoms, as sleep loss has been considered an important etiological factor in postpartum psychosis (
26).
For patients whose manic or psychotic symptoms persist after 3 days of benzodiazepine monotherapy, the next recommended step involves antipsychotic medication beginning on day 4 (step 2). Although the efficacy of antipsychotics in the absence of mood stabilizers has been described in only three case reports, antipsychotics are frequently used worldwide as first-line treatment for patients with postpartum psychosis and mania (
15). Furthermore, antipsychotics are often considered the preferred pharmacological treatment option for acute mania outside the postpartum period (
27).
The step 2 treatment with a combination of benzodiazepines and antipsychotics is recommended for 2 weeks. If at 2 weeks there is no significant clinical response, then adjunctive lithium is recommended (step 3). One small open-label study and a case report have suggested that the combination of lithium and antipsychotics is more effective than antipsychotic monotherapy in patients with postpartum psychosis (
13,
14). Furthermore, studies have demonstrated efficacy of lithium in the prevention of postpartum psychosis (
28–
31).
Finally, in patients whose symptoms have not responded after 12 weeks of combination treatment with a benzodiazepine, an antipsychotic, and lithium, ECT is recommended (step 4). Case reports and case series have described positive treatment outcomes with ECT in women with treatment-refractory postpartum psychosis (
25,
32).
Method
Participants
The study was approved by the Institutional Review Board of the Erasmus Medical Center (Rotterdam, the Netherlands). All patients provided written informed consent. The study was performed at the Mother-Baby Unit of the Department of Psychiatry at the Erasmus Medical Center, a five-bed inpatient unit that specializes in the care of patients with severe psychopathology in the postpartum period. Women are given the option for admission together with their baby in a fully staffed nursery adjoining the unit (
33). Every patient admitted to the unit between August 2005 and June 2011 (N=200) was screened for study inclusion and assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID) (
34).
Patients 18–45 years of age with a diagnosis of postpartum psychosis were eligible for the study. As postpartum psychosis is not described as a distinct illness entity in DSM-IV-TR, we defined eligible subjects as those patients for whom the SCID interview generated any of the following diagnoses and required the specifier “onset postpartum”: depressive disorder with psychotic features, psychotic disorder not otherwise specified, brief psychotic disorder, or mania. The specifier “onset postpartum” requires that the onset of symptoms occur within 4 weeks after delivery.
Overall, 83 patients fulfilled the criteria for postpartum psychosis or mania. Of these, 15 patients had a history of psychosis or mania outside the postpartum period and therefore were excluded from our analyses. One patient was excluded because of substance abuse, and two patients declined participation. One patient was lost to follow-up after remission; we describe her illness course separately. In total, 64 patients with psychosis or mania limited to the postpartum period were evaluated weekly during admission and at 9 months postpartum.
Nonpharmacological Treatment
All women received nonpharmacological interventions to optimize mother-baby interaction (
35). These interventions included feedback from nursing staff, video-interaction guidance, and baby massage.
Pharmacological Treatment
Step 1.
All patients were initially treated with lorazepam at bedtime for 3 days.
Step 2.
For patients receiving lorazepam monotherapy who had persistent manic or psychotic symptoms, antipsychotic medication was recommended beginning on day 4. Our primary recommendation for antipsychotic treatment was haloperidol at 2–6 mg/day. For patients in whom side effects occurred, we switched to an atypical antipsychotic. For a subset of patients (N=11) who had already been treated with an antipsychotic for more than 2 days before admission (e.g., by acute services), we skipped step 1 and continued treatment with the same antipsychotic.
Step 3.
After 2 weeks of combination treatment with a benzodiazepine and an antipsychotic, adjunctive lithium was recommended for those patients who did not have a significant clinical response. Lithium dosing was achieved based on plasma level (target, 0.8–1.2 mmol/L).
Step 4.
For patients who did not have a response after 12 weeks on combination treatment with a benzodiazepine, an antipsychotic, and lithium, ECT was recommended. All psychotropic medications would be tapered to discontinuation before initiation of ECT.
Maintenance Treatment
After complete remission of symptoms, all women were advised to taper benzodiazepines to discontinuation. Women receiving antipsychotic monotherapy were advised to continue this treatment as maintenance therapy until 9 months postpartum. Women who achieved clinical remission using both antipsychotics and lithium were advised to gradually taper off antipsychotic treatment, with maintenance lithium monotherapy until 9 months postpartum. Lithium dosing for relapse prevention was achieved based on plasma level (target, 0.6–0.8 mmol/L).
All women were encouraged to continue maintenance monotherapy throughout the first 9 months postpartum. Women who remained clinically stable after 9 months were assisted in gradually tapering their medication to discontinuation.
Assessments
In addition to the SCID, all participants and their relatives were interviewed by a psychiatrist (V.B. or K.M.K.). Duration of episode was defined as the number of days from the initial onset of psychiatric symptoms until remission. Phenomenology was quantified using the Bipolar Affective Disorder Dimension Scale (
36), a dimensional rating scale intended for use in clinical cohorts with a high incidence of bipolar spectrum illness. There are four identified dimensions: mania, depression, psychosis, and mood incongruence. During hospitalization, clinical evaluation was performed weekly using the Young Mania Rating Scale (
37), the Edinburgh Postnatal Depression Scale (
38), and the Clinical Global Impressions–Bipolar Disorder scale (CGI-BP), a modified version of the CGI scale that allows the clinician to rate the global illness severity and time course in patients with bipolar spectrum disorders (
39).
Clinical remission was defined as the absence of psychotic, manic, and depressive symptoms for at least 1 week, with a CGI-BP score ≤3, a Young Mania Rating Scale score ≤8, and an Edinburgh Postnatal Depression Scale score ≤10 (
40). We defined relapse as the occurrence of any mood or psychotic episode fulfilling DSM-IV-TR criteria or a CGI-BP score >3. Accordingly, sustained remission was defined as absence of any DSM-IV-TR mood or psychotic episodes throughout the follow-up period, as well as maintaining a CGI-BP score ≤3. Longitudinal assessment of mood episodes was performed using the National Institute of Mental Health Life-Chart Method at 8 months postpartum (
41).
In our data analysis, categorical demographic variables were compared with Fisher’s exact test, and continuous demographic variables were compared with the Mann-Whitney U test. Categorical outcomes of relapse risks were examined using Fisher’s exact test, logistic regression analysis, and Kaplan-Meier estimation of the log-rank test. Analyses were performed using SPSS, version 20.0 (IBM, Armonk, N.Y.). A two-tailed p value <0.05 was considered statistically significant.
Results
We assessed treatment outcomes for 64 enrolled patients. Participants’ demographic and clinical characteristics are summarized in
Table 1. The phenomenological classification included manic-psychotic features (65.6%), mixed episode (17.2%), and depression with psychotic features (9.4%). Only five patients had a postpartum psychosis without prominent affective symptomatology (7.8%). The median onset of initial psychiatric symptoms occurred at 8 days postpartum (interquartile range [IQR]=5–14). The median time to clinical remission was 40 days (IQR=26–65).
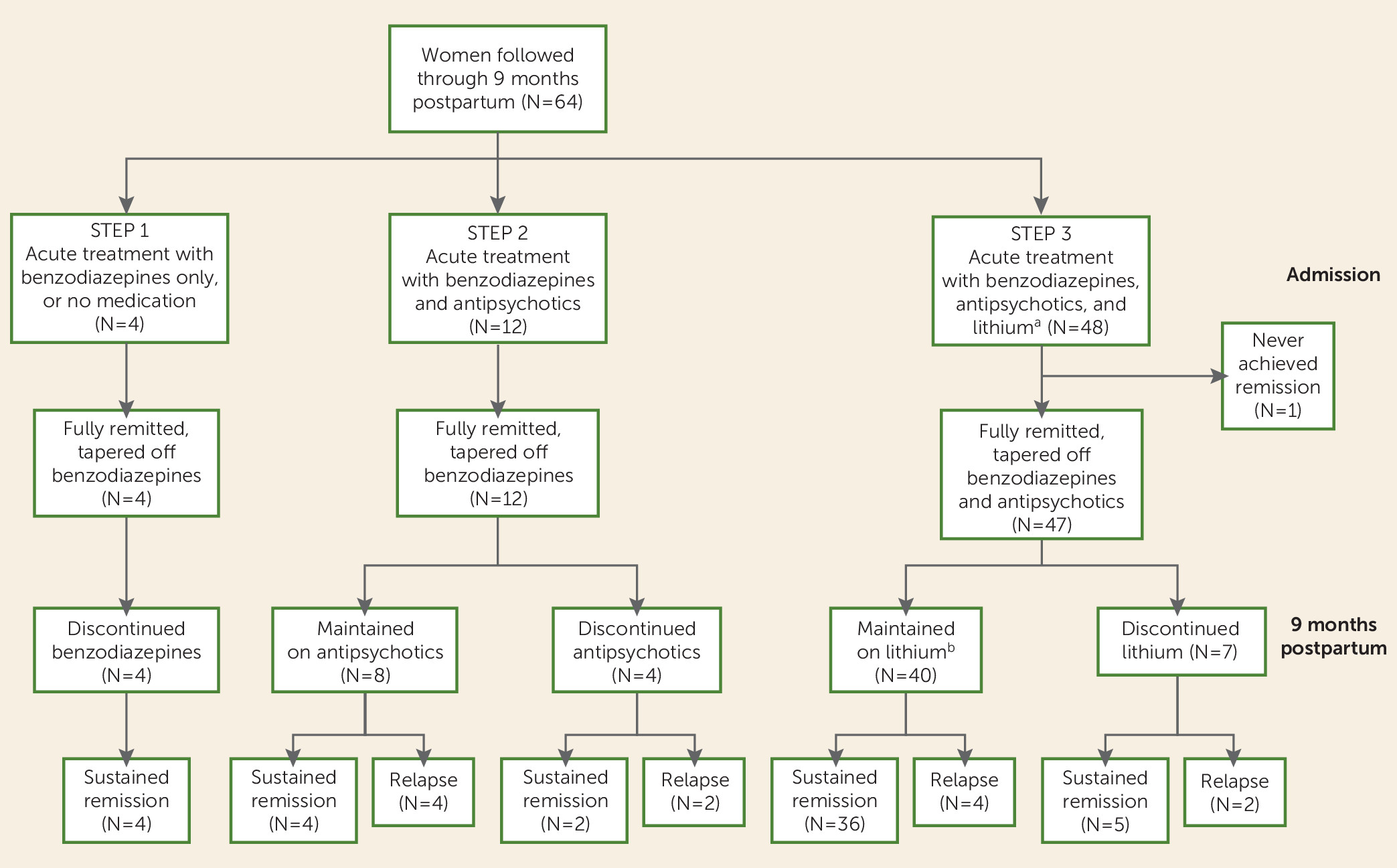
A flowchart of treatment outcomes is presented in
Figure 1.
Complete Remission
Four of the 64 patients (6.3%) remitted at step 1 of the treatment algorithm. Two of these patients experienced remission of manic and psychotic symptoms after only 3 days of benzodiazepine treatment (median time to complete remission, 21 days). Two patients declined to advance further in the treatment algorithm, despite having persistent symptoms at the end of the 3-day step 1 period. As expected, they had a substantially longer duration of illness, although they both ultimately achieved full remission (median time to remission, 161 days).
Twelve patients (18.8%) remitted during treatment with a combination of benzodiazepines and antipsychotics (step 2). Of these, seven were treated with haloperidol, four with olanzapine, and one with quetiapine. Ten of these patients experienced full resolution of manic and psychotic symptoms within 2 weeks of treatment initiation, despite a more persistent affective instability (median time to remission, 30 days; IQR=21–41). The remaining two patients were limited to step 2 despite persistent symptoms: one declined adjunctive lithium, and in the other patient lithium was contraindicated because of psoriasis (median time to remission, 39 days).
Forty-eight patients were treated with a combination of benzodiazepines, antipsychotics, and lithium. Of these, 47 patients (73.4%) remitted under step 3 of the treatment algorithm (
Figure 1). Antipsychotic treatment was as follows: 37 patients were treated with haloperidol, nine with olanzapine, and one with quetiapine. Of the 37 patients treated with haloperidol, eight were switched to an atypical antipsychotic because of side effects (five were switched to olanzapine, two to quetiapine, and one to risperidone). The remaining patient advanced from step 1 directly to step 3 after declining antipsychotic treatment and was therefore treated with only benzodiazepines and lithium. The median time to remission for patients treated under step 3 was 44 days (IQR=26–69).
Of the 48 patients treated with lithium, four were diagnosed as having clinical thyroid dysfunction at 9 months postpartum, compared with two patients found to have clinical thyroid dysfunction among the 16 who were not treated with lithium. All patients with clinical thyroid dysfunction were specifically diagnosed with autoimmune thyroid disease, as evidenced by thyroperoxidase antibody positivity. Five of the lithium-treated patients had transient subclinical thyroid dysfunction, for which no treatment was needed. Of these, three patients were thyroperoxidase antibody positive. Notably, none of the lithium-treated patients without autoimmune thyroid disease developed clinical thyroid dysfunction. There were no other adverse consequences of lithium treatment.
In total, 63 of the 64 enrolled patients (98.4%) achieved a full clinical remission within the first three steps of the clinical algorithm (
Figure 1). The remaining patient was discharged against medical advice during step 3 and did not achieve remission during the 9-month follow-up period. None of the 64 enrolled patients were treated with ECT (step 4). However, the one patient who was lost to follow-up did not respond to the sequential addition of benzodiazepines, antipsychotics, and lithium and remitted only after ECT treatment. This patient suffered from depression with psychotic features and was without manic symptoms. Her psychiatric symptoms began at postpartum day 4; the duration of illness was 186 days, after which the patient was discharged home and subsequently lost to follow-up.
We compared the demographic and clinical characteristics of patients who achieved clinical remission at step 1, 2, or 3. Women who received a combination of benzodiazepines and antipsychotics (step 2) were significantly older on average than those who received adjunctive lithium treatment in step 3 (Fisher’s exact test, p<0.01). No other differences were identified in demographic characteristics, psychiatric history, phenomenological characteristics, or postpartum latency to onset of symptoms.
Sustained Remission and Relapse Rates After 9 Months
Sustained remission at 9 months postpartum was observed in 51 of the 64 patients (79.7%) (
Figure 1). Of the remaining 13 patients, 12 relapsed (18.8%) and one had not remitted by 9 months postpartum (1.6%). Among the 12 patients who experienced relapse, 10 had a depressive episode (83.3%), one had manic symptoms without psychosis (8.3%), and one had a nonaffective psychotic episode (8.3%). Relapse occurred a median of 54 days after full remission (IQR=23–101). The median duration of the relapse episode was 61 days (IQR=30–73).
There were no differences in age, ethnicity, education, psychiatric history, or onset or duration of episode between patients who had a sustained remission (N=51) and those who did not (N=13) (
Table 1). Sustained remission was, however, associated with parity; primiparous women were more likely to achieve sustained remission (N=43/50, 86.0%) compared with multiparous patients (N=8/14, 57.1%) (Fisher’s exact test, p=0.03; odds ratio=4.6, 95% CI=1.2–17.4). Among the six multiparous patients who experienced relapse, two had a single previous episode of postpartum psychosis, and four had two previous episodes of postpartum psychosis.
With regard to phenomenology, patients with affective psychosis were more likely to achieve sustained remission (N=49/59, 83.1%) compared with those with nonaffective psychosis (N=2/5, 40.0%) (Fisher’s exact test, p=0.05; odds ratio=7.4, 95% CI=1.1–49.8). Among patients with affective postpartum psychosis, sustained remission was not associated with manic, mixed, or depressed symptomatology, nor with the mood congruence of psychotic symptoms.
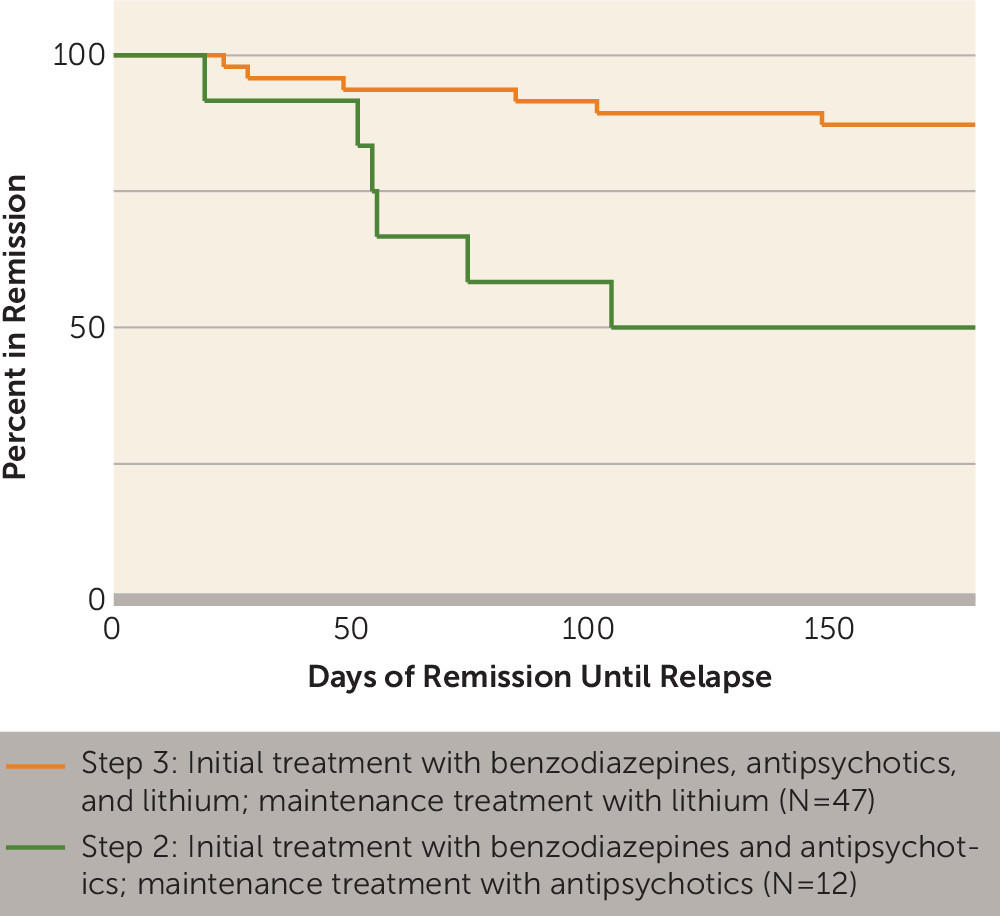
Effect of Medication on Relapse Rates
Treatment received was highly predictive of sustained remission (log-rank test, p=0.002) (
Figure 2). None of the patients treated with benzodiazepine monotherapy relapsed (N=4). Patients treated with the combination of benzodiazepines and antipsychotics (step 2) were significantly more likely to relapse (N=6/12, 50.0%) compared with those receiving adjunctive lithium in step 3 (N=6/47, 12.8%) (Fisher’s exact test, p=0.01; odds ratio=6.8, 95% CI=1.7–28.3).
We also evaluated whether noncompliance or medication discontinuation might have influenced the significantly higher rate of sustained remission in patients treated with adjunctive lithium. Among the 12 patients who remitted with benzodiazepines and antipsychotics under step 2, eight were continuously maintained on antipsychotics throughout the follow-up period, and four of these patients relapsed (N=4/8, 50.0%). Similarly, of the four patients who discontinued antipsychotic treatment, two relapsed (N=2/4, 50.0%). Therefore, relapse was not associated with antipsychotic discontinuation during the follow-up period.
Among patients who received adjunctive lithium (step 3), the majority (N=40/48, 83.3%) were continuously maintained on lithium throughout the follow-up period. Of these 40 patients, four relapsed (10.0%); their mean lithium level during follow-up (0.57 mmol/L, SD=0.41) was similar to that of patients who had sustained remission (0.58 mmol/L, SD=0.27). Eight patients discontinued lithium treatment during the follow-up period; of these, one never achieved remission and two relapsed (37.5%) (Fisher's exact test, p=0.08; odds ratio=5.4, 95% CI=0.9–31.6).
Discussion
Given that postpartum psychosis is a severe, potentially life-threatening disorder during the acute phase, the prognosis is remarkably good. Using a four-step clinical treatment algorithm, we observed high remission rates in the acute phase (98.4%), and a considerable proportion of patients achieved sustained remission (79.7%). The majority of patients required combination treatment with a benzodiazepine, an antipsychotic, and lithium to achieve clinical remission. Moreover, the rate of sustained remission was higher for patients using maintenance monotherapy with lithium than for those using maintenance monotherapy with an antipsychotic.
All but one patient achieved full clinical remission. The median duration of illness (from onset of postpartum psychosis until remission) was 40 days. This finding might suggest that the four-step treatment algorithm was highly effective. On the other hand, it might also be attributed to a spontaneously remitting illness course of relatively short duration. We and others have described a higher biological vulnerability during the postpartum period, including transient immunological and endocrine alterations (
42,
43). Therefore, even in the absence of treatment, substantial recovery might occur as a result of the spontaneous resolution of these transient changes in postpartum physiology. However, previous studies of medication-free patients do not support this alternative hypothesis. In the absence of pharmacological treatment, patients experience a substantially longer duration of illness until clinical remission (8 months on average) (
44). Moreover, in the present study, the two patients who declined antipsychotic and lithium treatment experienced a much longer duration of illness (>5 months), compared with the median duration of illness in the full cohort (40 days).
Overall, the naturalistic design of our study warrants a cautious interpretation of the effectiveness of each treatment step. Allowing a 3-day period of benzodiazepine treatment (step 1) before the initiation of antipsychotics (step 2) enabled us to carefully evaluate the influence of sleep hygiene on the severity of symptoms, given that restoration of sleep might lead to recovery in a subgroup of patients. Indeed, two patients responded promptly to benzodiazepine monotherapy. If antipsychotic treatment had been initiated sooner, the recovery of these two patients may well have been falsely attributed to the use of antipsychotics, and maintenance antipsychotic treatment would likely have been recommended.
The majority of patients responded to adjunctive lithium treatment and achieved clinical remission. Our results provide strong evidence that lithium is highly beneficial for acute treatment when given together with antipsychotics. However, it remains an open question whether this benefit would be fully realized with lithium monotherapy, or whether combination treatment with an antipsychotic is required. Future studies should also consider the potential benefits of initiating lithium earlier in the algorithm, in particular for women with postpartum psychosis and either a depressive or a mixed episode without severe manic or psychotic features. In contrast, it appears likely that the combination of lithium and an antipsychotic is the optimal strategy for the acute treatment of women with postpartum psychosis who have prominent manic or psychotic symptoms, given the proven benefits of antipsychotics over lithium monotherarpy for the treatment of acute mania or psychosis. As for antipsychotic options, our study was not designed to compare conventional and atypical antipsychotics, but this is undoubtedly another important question that should be addressed in future studies.
Lithium monotherapy was highly protective against relapse compared with antipsychotic monotherapy. Although lithium has not been described previously as a first-line option for maintenance therapy after postpartum psychosis, there is extensive evidence of the benefits of lithium for maintenance treatment in bipolar patients (
45). We previously reported evidence that rebound activation of the immune system during the postpartum period may be central to the pathogenesis of postpartum psychosis (
43). Accordingly, it is tempting to speculate that the immune suppressive action of lithium may have contributed to our positive treatment outcomes.
ECT has been reported to accomplish a swift reduction of symptoms in postpartum psychosis (
23–
25). In our study, severe symptoms such as agitation, mania, and psychosis responded well to pharmacotherapy, and a prolonged illness course was mostly due to affective instability. Remarkably, only one patient with depression with psychotic features was refractory to pharmacotherapy and required ECT treatment.
An overwhelming majority of our patients with postpartum psychosis were primiparous, which is consistent with previous studies describing primiparity as a significant covariate of postpartum psychosis (
33,
46–
48). Unexpectedly, we identified multiparity as a significant risk factor for relapse, as six of 14 multiparous women relapsed. Of note, this multiparous group included patients with a previous history of postpartum psychosis (N=10/14, of whom four relapsed). Accordingly, the threshold for manifesting clinical mood symptoms might be lower in these patients as a consequence of past episodes of postpartum psychosis, a phenomenon known as the “kindling hypothesis of mood disorders” (
49). However, the relapse rate of 50% among those multiparous patients who had no previous postpartum episodes is not explained by this hypothesis, although the numbers are too small (two of four patients) to establish firm conclusions.
Psychotic symptoms in the absence of affective symptoms were also identified as a significant risk factor for relapse. Notably, affective symptoms were present in >90% of our participants. There was no difference in relapse risk for patients with manic-psychotic symptoms (N=42) compared with patients with depressed-psychotic or mixed-psychotic symptoms (N=17). However, patients with nonaffective psychosis (N=5) had a significantly poorer prognosis, with a 60% relapse rate, compared with only 15% for patients with affective psychosis. Remarkably, affective symptoms were present in 12 of the 13 patients who relapsed (92.3%). Together, these findings contribute novel and compelling evidence to a broadening consensus that postpartum psychosis should be classified as an affective disorder and not a primary psychotic disorder (
31,
47).
The illustrative case vignette we present serves to make additional points about the diagnostic classification for patients with postpartum psychosis. Ms. B was primiparous, had no psychiatric history, and had prominent affective symptoms (mania). After remission, she has remained stable throughout 4 years of follow-up. According to both DSM-IV-TR and DSM-5 criteria, Ms. B should be diagnosed as having bipolar I disorder. However, this diagnosis suggests a lifelong vulnerability to manic and depressive episodes, whereas in fact some patients may have a biological vulnerability to severe affective psychosis that is limited to the postpartum period.
The postpartum period is well established as having a dramatically elevated risk for affective instability and psychosis; the risk during the postpartum period is estimated to be 20–25 times higher than during other periods (
1). Moreover, several studies have demonstrated that over long follow-up periods, a sizable proportion of women show no evidence of mania or psychosis outside the postpartum period (
4). Therefore, we suggest that Ms. B would be better served by a distinct diagnostic category of “postpartum mania,” for which the need for lifelong mood stabilization treatment is currently unknown. Naturally, however, Ms. B’s diagnosis should be converted to bipolar disorder should she ever meet the diagnostic criteria outside the postpartum period.
Summary and Conclusions
In this study, we found that patients with postpartum psychosis or mania achieved favorable treatment outcomes using a structured treatment algorithm during the acute phase of the illness. After remission, maintenance with lithium monotherapy appears to be highly protective against relapse.
Based on observations from our case series and the existing literature, we propose the recommendations below for the treatment of patients with postpartum psychosis.
General Strategies
•
Inpatient psychiatric treatment is essential to ensure the safety of mother and baby. Admission to a mother-baby unit is associated with improved patient satisfaction and may help reduce time to recovery (
50).
•
The clinician must inquire about any thoughts the patient might have of harming herself or her children.
•
The initial clinical evaluation for postpartum psychosis requires a thorough medical and psychiatric history, including physical and neurological examinations. Laboratory testing should include a complete blood count, liver function tests, and levels of electrolytes, blood urea nitrogen, creatinine, calcium, and glucose. A urine drug screen should also be performed. We also suggest measuring thyroid-stimulating hormone, free T4, and thyroid peroxidase antibodies, at the time of diagnosis as well as 6 months postpartum. With proper clinical indication, brain CT or MRI, CSF analysis, limbic encephalitis antibody screening, measurement of serum vitamin B1, B12, and folate levels, and urinalysis should also be performed.
•
Focus on sleep hygiene and a structured rhythm of feedings. In clinical practice, this often means cessation of breastfeeding. Use of lactation inhibitors should be avoided.
•
Mother-baby interaction deserves particular attention (
35,
51,
52). Moreover, support for the father is also an important aspect of successful treatment from the perspective of the family unit.
Pharmacotherapy
•
Allowing a short period (36–72 hours) of benzodiazepine treatment before initiation of antipsychotics enables the clinician to carefully evaluate the influence of sleep hygiene on the severity of symptoms while also screening for somatic comorbidities. Restoration of sleep may lead to recovery in a subgroup of patients.
•
We discourage the use of antidepressants for the acute treatment of postpartum depression with psychotic features, particularly in the absence of appropriate mood stabilization, because of the risk of exacerbating mood instability (
53,
54).
•
Lithium is highly recommended during the acute phase of the illness, unless otherwise contraindicated (e.g., because of impaired thyroid or kidney function).
•
Antipsychotics are recommended for the acute treatment of manic and psychotic symptoms.
•
We recommend maintenance treatment using lithium monotherapy during the first 9 months postpartum (target plasma level, 0.6–0.8 mmol/L). After 9 months, a gradual tapering off of lithium should be considered in patients who remain in full clinical remission.
ECT
•
The choice between pharmacotherapy and ECT should be made in consultation with the patient, particularly with regard to their preference for breastfeeding.
•
ECT has been described as a highly effective treatment option in patients with severe catatonic features (
23–
25).
•
ECT should be considered for treatment of postpartum depression with psychotic features, given the relatively longer median duration of illness compared with postpartum mania (
33).
Acknowledgments
The authors are grateful to Annemarie van Hulst and Monique Raats for guidance in developing a standardized treatment algorithm and Mirjam Timmermans, Jeroen Vervoort, and Siska Verploegh for their management and database assistance.