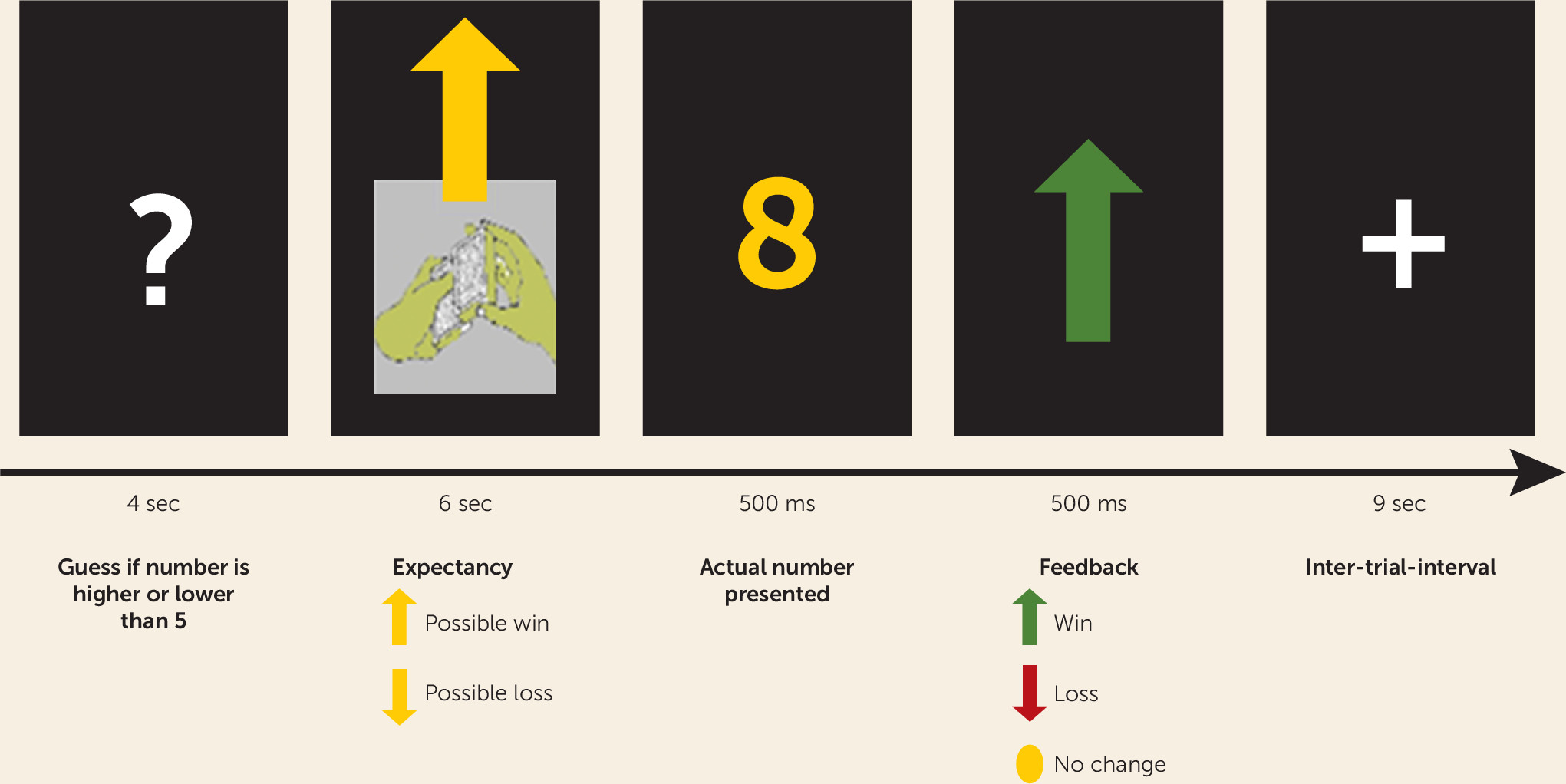
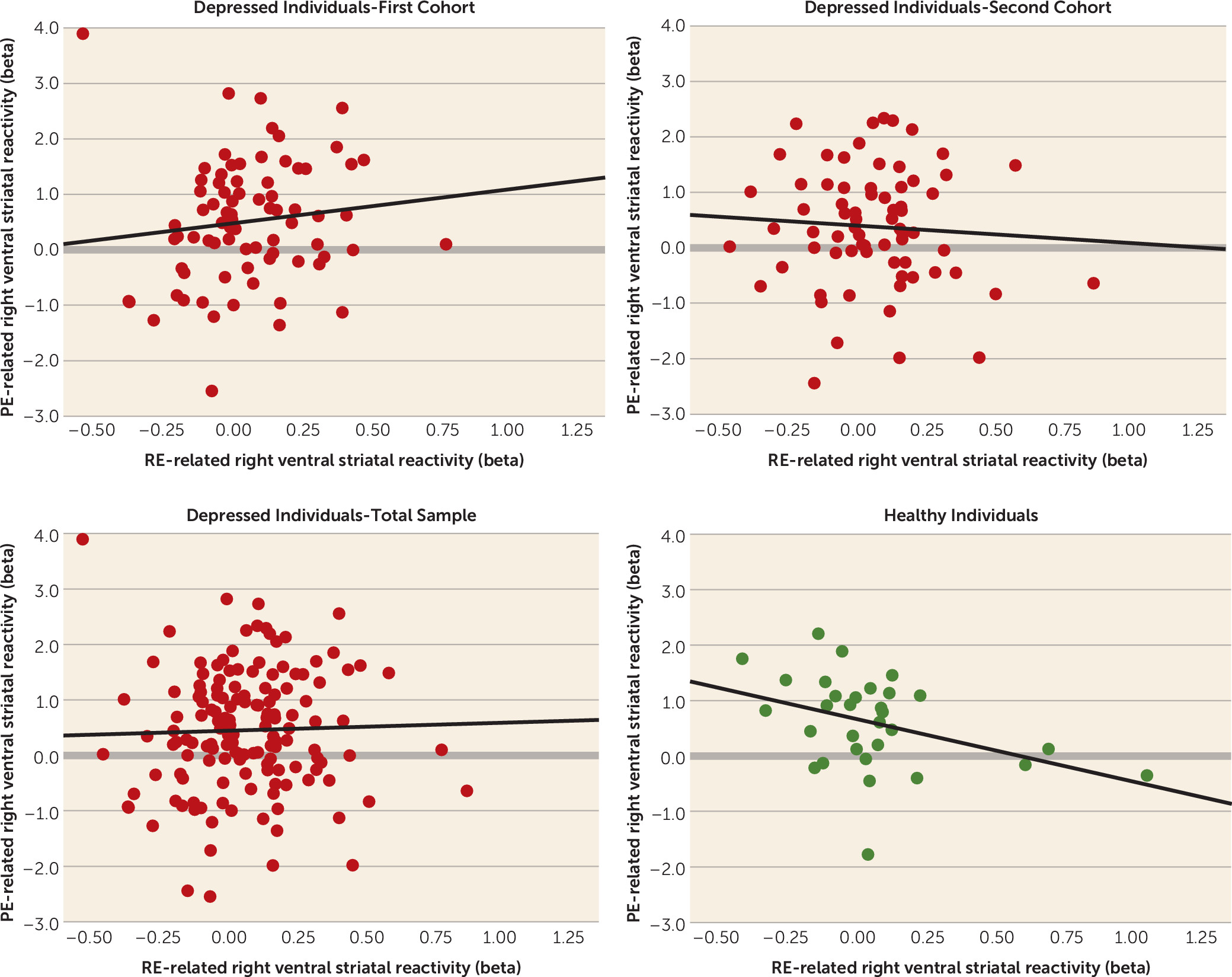
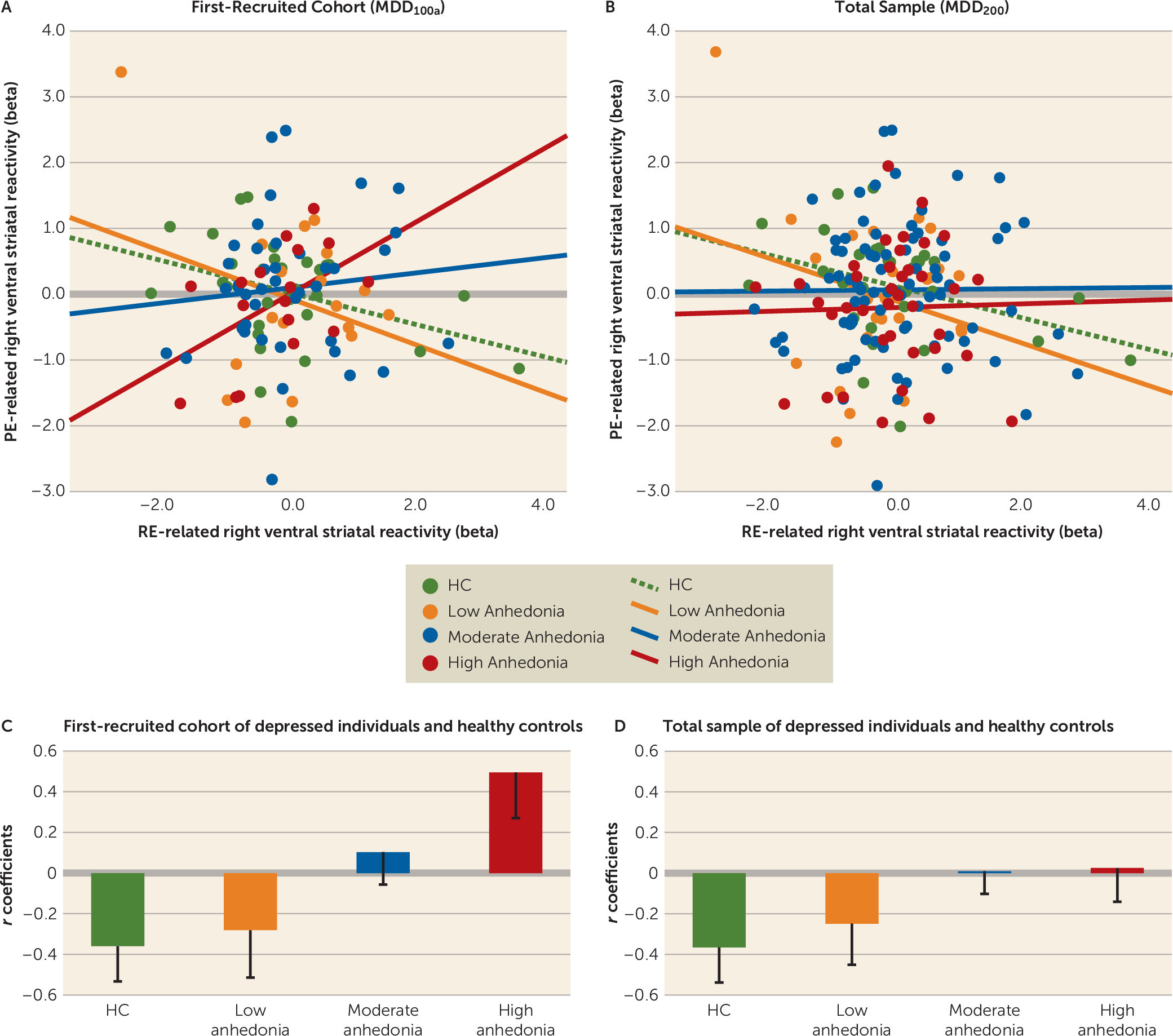
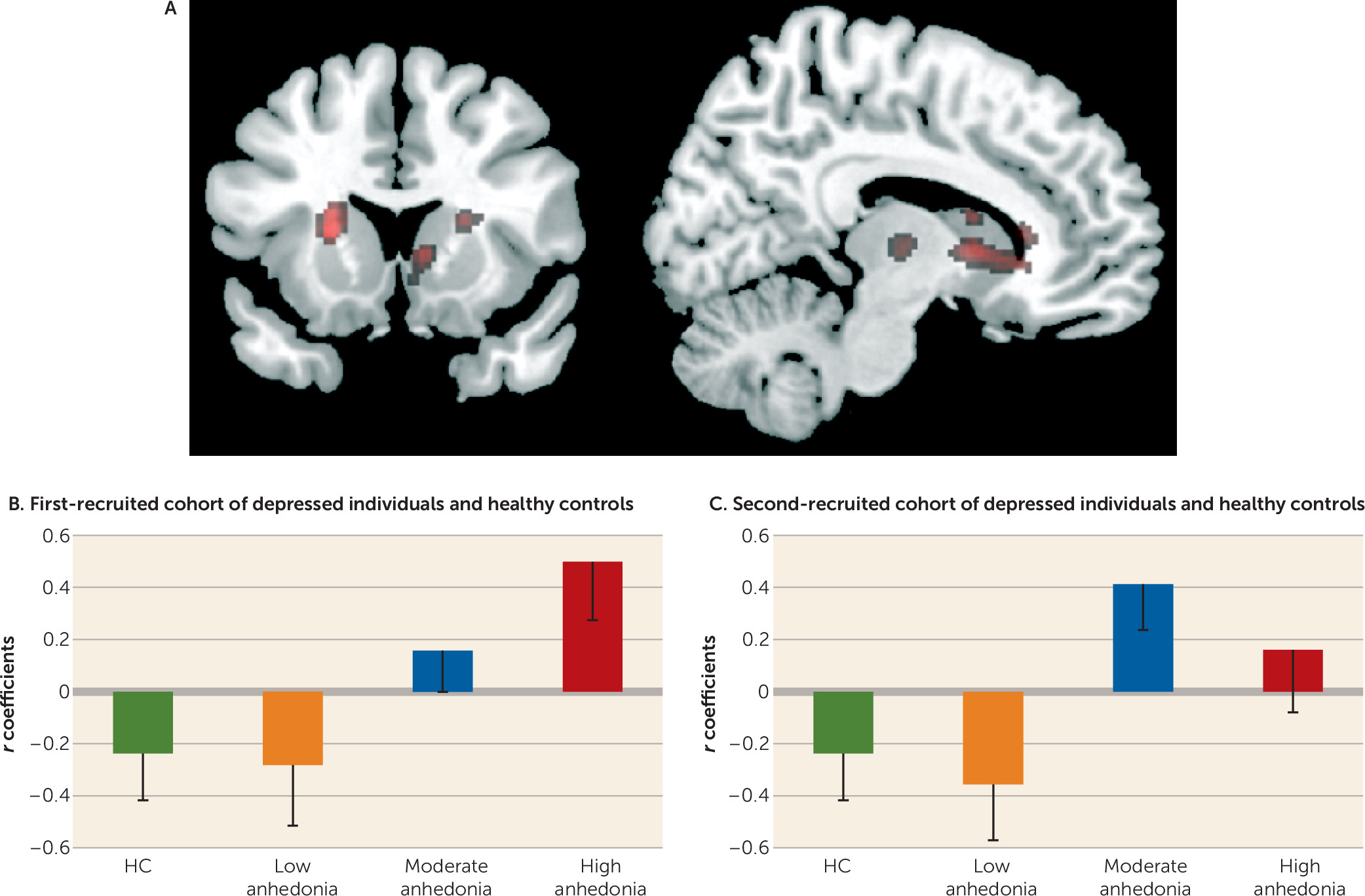
Competing Interests
Dr. Almeida has received support from the American Academy of Child and Adolescent Psychiatry Pilot Research Award for General Psychiatry Residents, supported by Pfizer. Dr. Deckersbach has received research funding from the Depressive and Bipolar Disorder Alternative Treatment study, the International OCD Foundation, NARSAD, NIMH, TSA, and Tufts University; he has received honoraria, consultation fees, and/or royalties from Boston University, BrainCells, Catalan Agency for Health Technology Assessment and Research, Clintara, LLC, the Massachusetts General Hospital Psychiatry Academy, the Massachusetts Medical Society, the National Association of Social Workers-Massachusetts, the National Institute on Drug Abuse, NIMH, Oxford University Press, Systems Research and Applications Corporation, and Tufts University; and he has participated in research funded by the Agency for Healthcare Research and Quality, Cyberonics, Forest Research Institute, Janssen Pharmaceuticals, Medtronic, NIA, NIH, Northstar, Shire Development, and Takeda. Dr. Toups has received travel funds from Janssen Research and Development and currently receives compensation for serving on a data safety and monitoring board for Otsuka Pharmaceuticals. Dr. Kurian has received research funding/grants from Evotec, Forest Pharmaceuticals, Johnson and Johnson, Naurex, NIMH, Pfizer, Rexahn, and Targacept. Dr. Phillips has received funding from NIMH and the Emmerling-Pittsburgh Foundation. Dr. Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale and has received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current study; she was the recipient of a grant from Eli Lilly to support a year’s salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D.; she has received unrestricted educational grants and/or lecture fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Otsuka, Pfizer, Sanofi-Aventis, and Shire; and her family owns stock in Bristol-Myers Squibb. Dr. McGrath has received research grant support from Forest, Naurex, and Sunovion. Dr. Fava has received research support from Abbot Laboratories, Alkermes, American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir Pharmaceuticals, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clintara, LLC, Covance, Covidien, Eli Lilly, EnVivo Pharmaceuticals, Euthymics Bioscience, Forest Pharmaceuticals, Ganeden Biotech, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen Research and Development, LLC, Jed Foundation, Johnson and Johnson Pharmaceutical Research and Development, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbec, MedAvante, Methylation Sciences, NARSAD, the National Center for Complementary and Alternative Medicine, the National Institute of Drug Abuse, NIMH, Neuralstem, Novartis AG, Organon Pharmaceuticals, Pamlab, LLC, Pfizer, Pharmacia-Upjohn, Pharmaceutical Research Associates, Pharmavite, LLC, PharmoRx Therapeutics, Photothera, Reckitt Benckiser, Roche Pharmaceuticals, RCT Logic, LLC (formerly Clinical Trials Solutions, LLC), Sanofi-Aventis US LLC, Shire, Solvay Pharmaceuticals Stanley Medical Research Institute, Synthelabo, and Wyeth-Ayerst Laboratories; he has received advisory/consulting fees from Abbott Laboratories, Affectis Pharmaceuticals AG, Alkermes, Amarin Pharma, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, BioMarin Pharmaceuticals, Biovail Corporation, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, CNS Response, Compellis Pharmaceuticals, Cypress Pharmaceutical, DiagnoSearch Life Sciences (P) Ltd., Dinippon Sumitomo Pharma Co., Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, GenOmind, LLC, GlaxoSmithKline, Grunenthal GmbH, i3 Innovus/Ingenis, Janssen Pharmaceutica, Jazz Pharmaceuticals, Johnson and Johnson Pharmaceutical Research and Development, LLC, Knoll Pharmaceuticals Corp., Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, Naurex, Neuralstem, Neuronetics, NextWave Pharmaceuticals, Novartis AG, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, LLC, Pfizer, PharmaStar, Pharmavite LLC, PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, LLC ( formerly Clinical Trials Solutions, LLC), Rexahn Pharmaceuticals, Ridge Diagnostics, Roche, Sanofi-Aventis US LLC, Sepracor, Servier Laboratories, Schering-Plough Corporation, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical Company Limited, Tal Medical, Tetragenex Pharmaceuticals, TransForm Pharmaceuticals, Transcept Pharmaceuticals, and Vanda Pharmaceuticals; he has received speaking/publishing fees from Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, Imedex, Massachusetts General Hospital Psychiatry Academy/Primedia, Massachusetts General Hospital Psychiatry Academy/Reed Elsevier, Novartis AG, Organon Pharmaceuticals, Pfizer, PharmaStar, United BioSource, and Wyeth-Ayerst Laboratories; he is a shareholder with Compellis and PsyBrain; and he has received patents/other income/royalties from the following: patent for Sequential Parallel Comparison Design (licensed by Massachusetts General Hospital Psychiatry to Pharmaceutical Product Development, LLC) and patent application for a combination of ketamine plus scopolamine in major depressive disorder, Copyright for the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire, Sexual Functioning Inventory, Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs and Symptoms, and SAFER, royalties from Lippincott, Williams and Wilkins, Wolters Kluwer, and World Scientific Publishing. Dr. Weissman has received funding from the Interstitial Cystitis Association, NARSAD, the National Institute on Drug Abuse, NIMH, the Sackler Foundation, and the Templeton Foundation; and she receives royalties from American Psychiatric Publishing, MultiHealth Systems, Oxford University Press, and Perseus Press. Dr. Trivedi is or has served as an advisor/consultant and has received fees from Abbott Laboratories, Abdi Ibrahim, Akzo (Organon Pharmaceuticals), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb, Cephalon, Cerecor, CME Institute of Physicians, Concert Pharmaceuticals, Eli Lilly, Evotec, Fabre Kramer Pharmaceuticals, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson and Johnson Pharmaceutical Research and Development, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Pfizer, PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories; and he has received grant/research support from the Agency for Healthcare Research and Quality, Corcept Therapeutics, Cyberonics, Merck, NARSAD, NIMH, and the National Institute on Drug Abuse. All other authors report no financial relationships with commercial interests.